Breaking News! Johnson & Johnson Medical's Flagship Product Sued
01
10 years' imprisonment
Recently, the U.S. Court of Appeals for the Federal Circuit overturned a ruling by the District Court of Massachusetts in a dispute over heart pump patents, determining that the district judge misunderstood the contested claim terms, which led to both parties reaching a 'non-infringement consensus' based on the incorrect interpretation.
The protagonists of the case are Abiomed of Johnson & Johnson and Maquet of Getinge.
The lawsuit can be traced back to December 2015, when Maquet, a subsidiary of the company J&J, sent a letter to Abiomed (now part of the MedTech department of Roche) in Massachusetts, accusing the Impella series of infringing on three patents and seeking a licensing agreement.
In 2016, Abiomed filed a lawsuit against Maquet for a declaratory judgment of non-infringement of patent rights. As the case progressed, counterclaims from both parties increased the number of disputed patents to six.
In 2017, Maquet sued Abiomed, alleging that its Impella heart pump infringed two other blood pump patents (Nos. 10,238,783 and 9,789,238), referred to as the 783 and 238 patents.
District Judge F. Dennis Saylor IV adopted the prosecution disclaimer proposed by Abiomed, concluding that the statements made by Maquet during the application for other related patents constituted a clear relinquishment of the scope of the disputed patent rights. Based on this interpretation, both parties had reached a consensus of "no infringement."
However, the Federal Circuit Court noted that the district court erroneously used Maquet's statements made when applying for other patents to interpret the scope of rights at issue in the patent at hand (No. 783). The court emphasized that a patent renunciation occurs only when the patent owner 'clearly and unambiguously renounces' (unequivocally disavowed) specific claims during the application process.
The case will be retried in the district court after the original case is reheard by the court of appeals. Maquet must prove that Abiomed's product infringes its patent.
It is worth noting that shortly after the local court made the original ruling, Johnson & Johnson announced its acquisition of Abiomed for $16.6 billion, which may cast a shadow of uncertainty over its subsequent operations due to this patent dispute.
02
$16.6 billion bet on an "artificial heart"
In November 2022, Johnson & Johnson announced its acquisition of Abiomed, a manufacturer of artificial hearts, for approximately $16.6 billion, marking its high-profile entry into the artificial heart market.
Artificial hearts can be classified into surgical implantation and interventional types based on the implantation method. Abbott dominates the field of left ventricular assist devices, while Johnson & Johnson's Abiomed "rules" in the field of percutaneous ventricular assist devices (pVAD).
As early as 1987, the target company Abiomed had already gone public on NASDAQ. It has experienced a remarkable turnaround from the brink of bankruptcy to significant growth, successfully stepping into the forefront of the global heart disease sector.
The Impella cardiac pump resembles a gray pen with stripes, featuring a slender wire at its tip. It is implanted into the patient's heart via a catheter introduced through the groin and connected to an automated Impella controller beside the patient's bed, providing continuous blood flow support to enable more efficient pumping by the heart, thereby improving cardiac function.
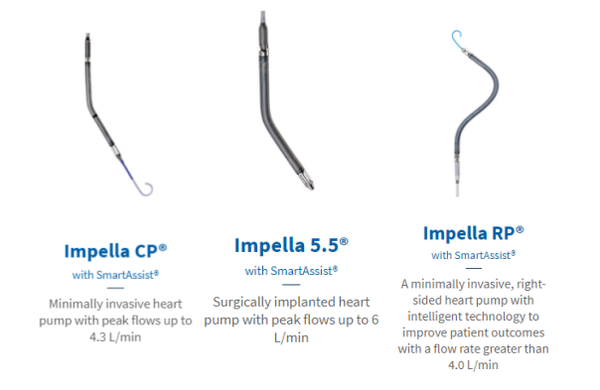
Source: Abiomed的官网
Down below, Abiomed possesses the world's only FDA-certified transcatheter cardiac assist device, with its Impella product line having few competitors in the global field of transcatheter heart pumps.
In 2023, Abiomed received three Class I recalls.
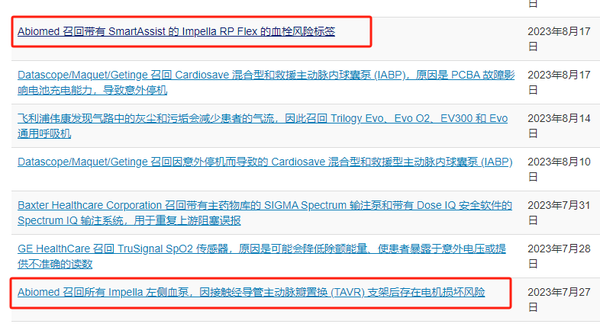
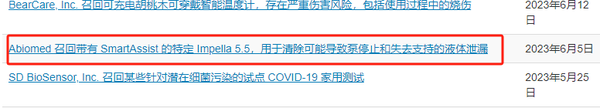
Source: FDA website
In 2024, following 49 fatalities, Abiomed initiated its largest recall in history, recalling six models of Impella heart pumps on a global scale. A total of 66,390 units were recalled in the United States, while over 26,000 units were recalled outside the U.S., with the combined total reaching 90,000 units.
Recall involves multiple countries including the United States, Japan, Canada, Germany, France, India, Mexico, and Taiwan in addition to China.
Amid the resumption of the patent dispute with Getinge, this cardiovascular giant is set to face new challenges.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






