Why A Small Amount of Plasticizer Makes PVC More Brittle: Understanding the Anti-Plasticizing Effect
PVC plasticizers do indeed exhibit the "antiplasticization effect." This is a seemingly counterintuitive yet crucial phenomenon in the application of plasticizers, and it is a "performance trap" that every PVC formulation engineer must be vigilant about.

1. What is the anti-plasticization effect?
Antiplasticization effectThis means: when added to PVC When plasticizers (usually 5-15 phr, i.e., 5-15 parts per hundred resin) are added, the material's rigidity, strength, and hardnessThe situation has not improved but worsened.at the same timeThe impact strength and elongation at break decreased significantly.The material becomes more... ...phenomenon.
This is completely contrary to our intuitive understanding that "plasticizer = softening." Only when the plasticizer content exceeds a certain critical value does the material begin to exhibit the expected softening and toughening trend.
Simply put:
0 portions of plasticizerHard and brittle pure PVCGlass state)
Add 5-15 parts of plasticizer.Become harder and more brittle!Anti-plasticization region)
Add >20-30 portions of plasticizer. Start to soften and become pliableNormal plasticizing zone)
2. The Microscopic Mechanism of Antiplasticization: "Molecular-Level Shackles"
To understand this "anomalous" phenomenon, we need to delve once again into the molecular world:
1. The "brittle hardness" of pure PVC:The strong intermolecular forces between pure PVC molecular chains cause the chain segments to be "frozen," which macroscopically results in high modulus (hardness) and low toughness (brittleness). Its brittleness stems from the inability of the chain segments to move, making it difficult to dissipate and absorb impact energy.
2. The "disruption" caused by a small amount of plasticizer:
When a small amount of plasticizer molecules insert between PVC chain segments, it is insufficient to Chain spacing, providing the "free volume" required for large-scale motion of chain segments.
On the contrary, these polar plasticizer molecules will act like "Molecular wedge"or"Crosslinking pointSimilarly, stronger interactions occur with the polar sites (chlorine atoms) on the PVC chain (such as dipole-dipole interactions or even hydrogen bonding, which are stronger than van der Waals forces).
The result is:Not only does it fail to lubricate and isolate, but it actually “reinforces” the already strong intermolecular forces, locking the molecular chains even tighter!The rigidity of the entire molecular network increases rather than decreases.
3. Segmental motion is further restricted.Under this "restriction" state, the local micro-Brownian motion ability of the chain segments is even worse than that of pure PVC. When the material is subjected to impact, the stress cannot be effectively dissipated through chain segment movement and can only concentrate at defects, leading to rapid crack propagation. is manifested as lower impact strength and elongation at breakIt is more brittle.
3. Comparison between the anti-plasticization region and the normal plasticization region!
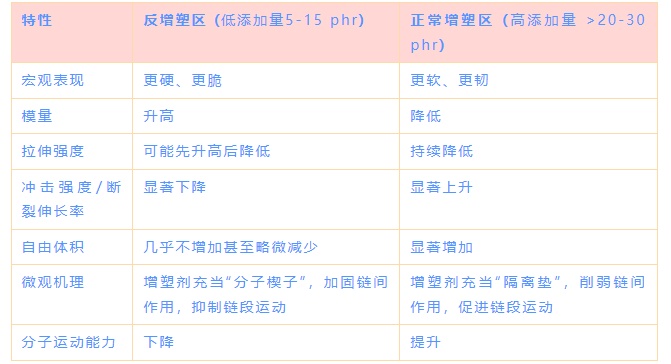
4. Why is the anti-plasticization effect crucial?
1. "Pitfalls" in Formula DesignIf a product needs to possess both a certain degree of rigidity and good toughness (such as some electronic casings or pipes), engineers may instinctively add a small amount of plasticizer to "fine-tune" the toughness. However, if they are unaware of this effect, they may inadvertently enter the "anti-plasticization region," resulting in a product that is actually less tough than if no plasticizer had been added. It is highly prone to brittle cracking during use, leading to product failure.
2. Key Aspects of Performance PredictionTo understand the anti-plasticization effect, one must be able to draw a complete PVC performance–plasticizer dosage curve. This graph does not start at the origin and decrease monotonically; instead, it first goes through a phase ofCamel hump (or depression)This is the scientific basis for guiding precise formula design.
PVC plasticizer's anti-plasticization effect diagram
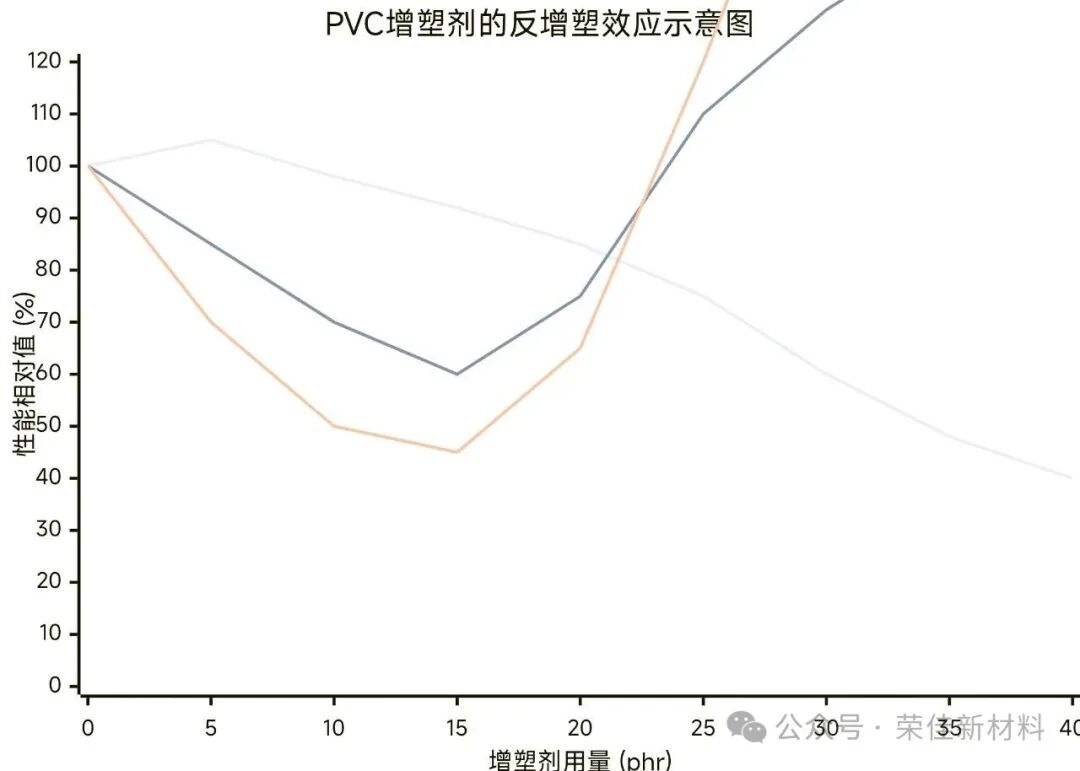
(Note: phr = parts per hundred resin, which refers to the number of parts per hundred parts of resin and is a commonly used unit in plastic formulations.)
This figure reveals the trends of three key performance indicators with varying amounts of plasticizer.
1. Modulus/Hardness(Modulus/Hardness)
Trend: When a small amount of plasticizer is added (5-15 phr), the modulus increases rather than decreases, reaching a peak value, and then continuously decreases as the amount increases further.
Interpretation: A small amount of plasticizer acts like a "molecular wedge," temporarily strengthening the intermolecular forces and increasing the material's rigidity.
Impact Strength/Toughness
Trend: This is the indicator most significantly affected by the anti-plasticization effect. Its curve presents a deep "valley," reaching the lowest point in the anti-plasticization region (10-15 phr), where the material becomes more brittle than pure PVC. After exceeding the critical value, the toughness rises sharply.
Interpretation: The segmental motion is completely suppressed, making it impossible to dissipate impact energy, resulting in brittle fracture.
3. Elongation at Break
Trend: Similar to the resilience curve, it first drops sharply to the bottom, and then rises significantly.
Interpretation: In the anti-plasticization region, the material has extremely poor ductility and breaks as soon as it is stretched; after normal plasticization, the chain segments can move freely, making the material very flexible and stretchable.
Core Conclusion:
Anti-plasticization zone(Antiplasticization Zone): The shaded area in the diagram represents the dangerous "performance trap zone." Within this range, the material exhibits counterintuitive embrittlement behavior.
Critical dosage(Critical Concentration): The plasticizer amount must exceed a certain critical value (usually about 15-20 phr) to enter the expected normal plasticizing region, where the material begins to become soft and tough.
3. Explain the abnormal phenomenon.The bizarre phenomenon of "becoming more brittle after adding a plasticizer" during production often stems from mistakenly entering the anti-plasticization region.
5. How to avoid the anti-plasticization effect?
1. Crossing the critical point:Decisively increase the amount of plasticizer to above the anti-plasticization region (typically >20 phr)....to ensure that it provides sufficient free volume for the necessary segmental mobility and achieves the intended plasticizing effect. If the product does not require such a high degree of softness, alternative modification methods (such as toughening with elastomers) should be considered, rather than adding a small amount of plasticizer in a cursory manner.
2. Selection of Plasticizer TypesDifferent plasticizers produce anti-plasticization effects.Different critical concentrationsGenerally, highly compatible plasticizers (such as phthalates) exhibit a more pronounced anti-plasticization effect. In contrast, some high molecular weight or slightly less compatible plasticizers may have a narrower or less noticeable anti-plasticization range.
Conclusion:
The antiplasticization effect profoundly reveals the complexity of the relationship between the structure and properties of polymer materials. It tells us that plasticizers are not simply "lubricants," but there exists a critical transition between "confinement" and "liberation" in their interaction with polymers.
For PVC formulators, remember one golden rule.:
Either don't add it, or add enough.Adding a small amount of plasticizer with great caution is likely not improving performance but rather forging a "molecular shackle" that makes the product brittle.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






