University of California Teams Up with Nature: Ethylene and Base Metal Heterogeneous Catalysts Convert Polyolefin Waste into Light Olefins
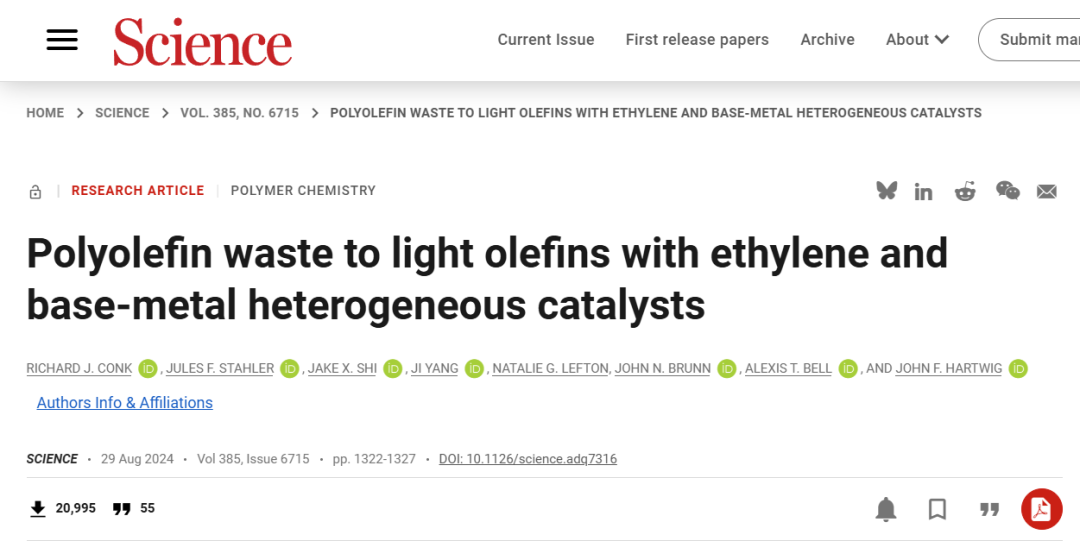
The selective conversion of polyethylene, polypropylene, and mixtures of these two polymers is urgently needed to produce products in high demand, as current methods suffer from low selectivity, generate large amounts of greenhouse gases, or rely on expensive disposable catalysts. The isomerization-ethylene cleavage of unsaturated polyolefins is an energy and environmentally feasible route to propylene and isobutylene. However, it currently requires precious metal homogeneous catalysts and unsaturated polyolefins, and the process is limited to polyethylene.
In this work, research by Professor John Hartwig, a member of the U.S. National Academy of Sciences and the American Academy of Arts and Sciences at the University of California, Berkeley, and Professor Alexis T. Bell, a member of the U.S. National Academy of Sciences, the National Academy of Engineering, and the American Academy of Arts and Sciences, demonstrates that a simple combination of tungsten oxide on silica and sodium on γ-alumina can convert polyethylene, polypropylene, or mixtures of both (including post-consumer forms of these materials) into propylene or a mixture of propylene and isobutylene at 320 °C with yields exceeding 90%, without the need for dehydrogenation of the starting polyolefins. The related findings were published in Science under the title "Polyolefin waste to light olefins with ethylene and base-metal heterogeneous catalysts."
Research Content
Na/γ-Al2O3 Catalytic Cracking of Polyolefins
The author studied the method of using heterogeneous catalysts to deconstruct polyolefins (PE and PP) into light olefins, focusing on addressing the challenge of the initial dehydrogenation step. By heating polyolefins with a Na/γ-Al2O3 catalyst at 320 °C, the author successfully promoted the cleavage of polymer chains and the formation of olefins containing C=C bonds, thereby providing a foundation for the subsequent isomerization ethylene decomposition (IE) process. Experimental results showed that under these conditions, the molecular weight of the polymers significantly decreased, producing shorter polymer chains and olefins. Compared with the control without catalyst, the Na/γ-Al2O3 catalyst demonstrated a clear advantage in chain scission and olefin generation.
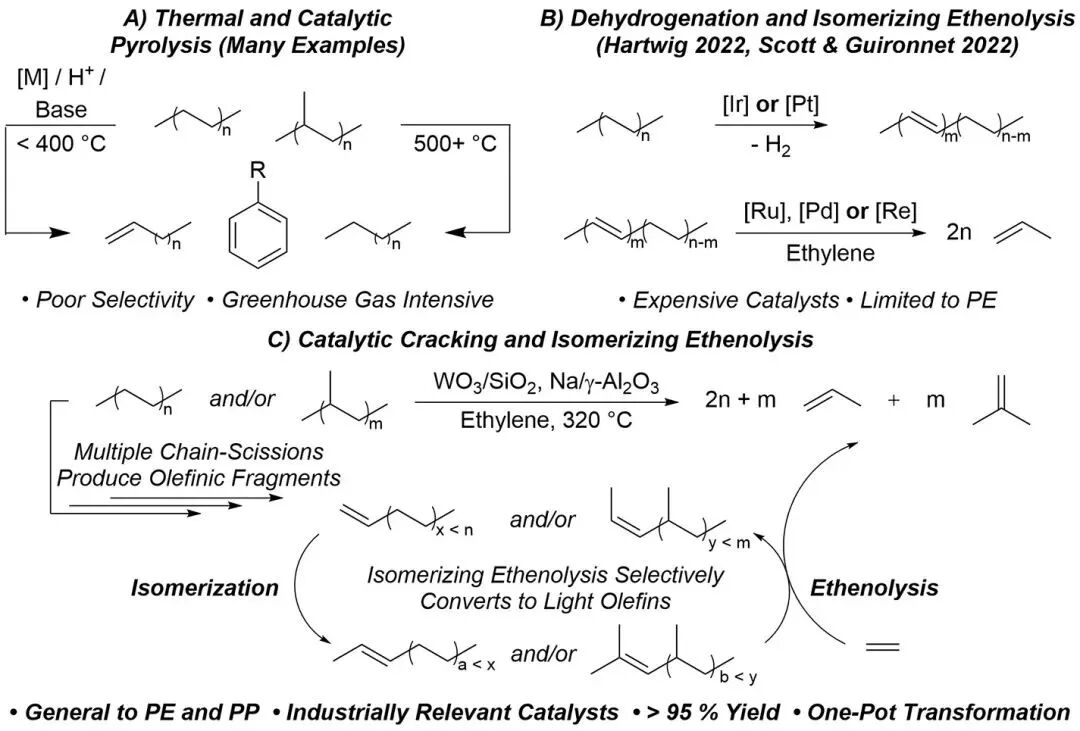
Figure 1 Comparison with Existing Technology
WO3/SiO2-Catalyzed Polyolefin Cracking and Olefin Metathesis
In the study, the authors found that Na/γ-Al2O3 effectively catalyzed the cracking of PP and PE into long-chain olefins at 320 °C. Building on previous work on the isomerization effect of this catalyst on olefins, the authors explored the effectiveness of using WO3/SiO2 as an olefin metathesis catalyst. At 320 °C, WO3/SiO2 successfully catalyzed the cracking of iPP, producing 11.8% olefins, which is three times the amount produced when using Na/γ-Al2O3. In contrast, HDPE produced fewer internal olefins under the same conditions, indicating that the cracking of iPP is more significant under the catalysis of WO3/SiO2, while the chain scission and olefin generation of PE are less pronounced.
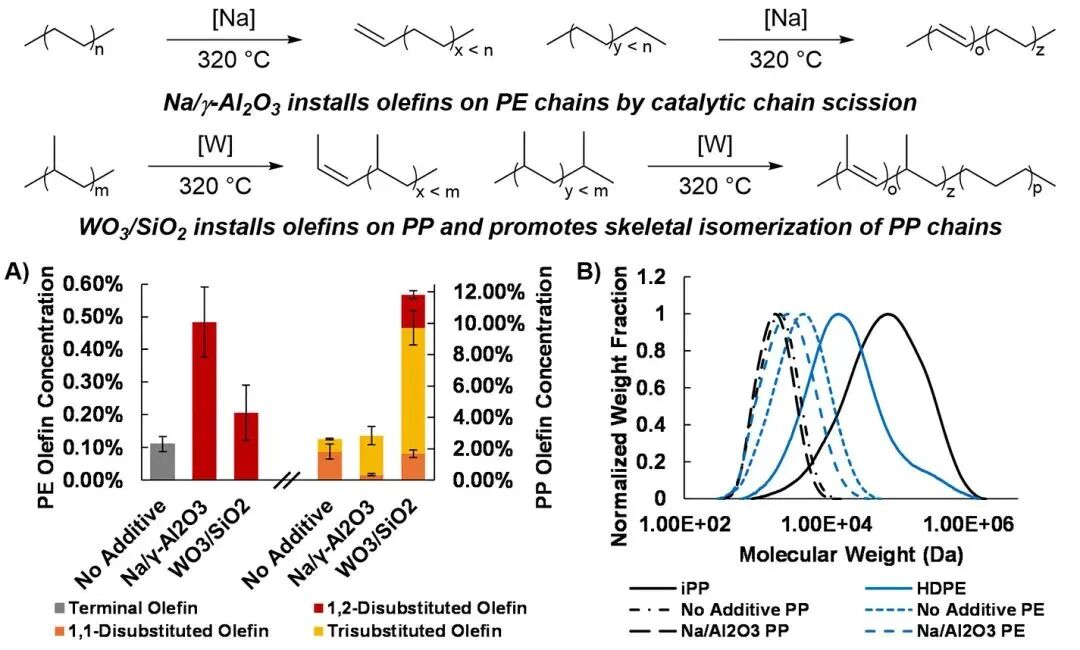
Figure 2 Polyolefin Catalytic Cracking Experiment
Polypropylene, polyethylene, and
CIE of a mixture of polypropylene and polyethylene
The authors found that combining WO3/SiO2 with Na/γ-Al2O3 could efficiently catalyze the cracking of PP and PE into light olefins under 320 °C and 15 bar ethylene pressure. PP was converted into a mixture of propylene and isobutene with a ratio of about 2:1 (Figure 3A), while PE was mainly converted into propylene with a yield as high as 87% (Figure 3B). This reaction mechanism involves the isomerization of the polypropylene main chain, converting -CH(Me)- units into -CH2CH2- units, resulting in a propylene yield that exceeds the stoichiometric prediction. This method also showed high efficiency in converting a mixture of HDPE and PP into propylene and isobutene, with a propylene-to-isobutene ratio of 5.7:1, indicating the potential application of this catalytic system in mixed waste streams.
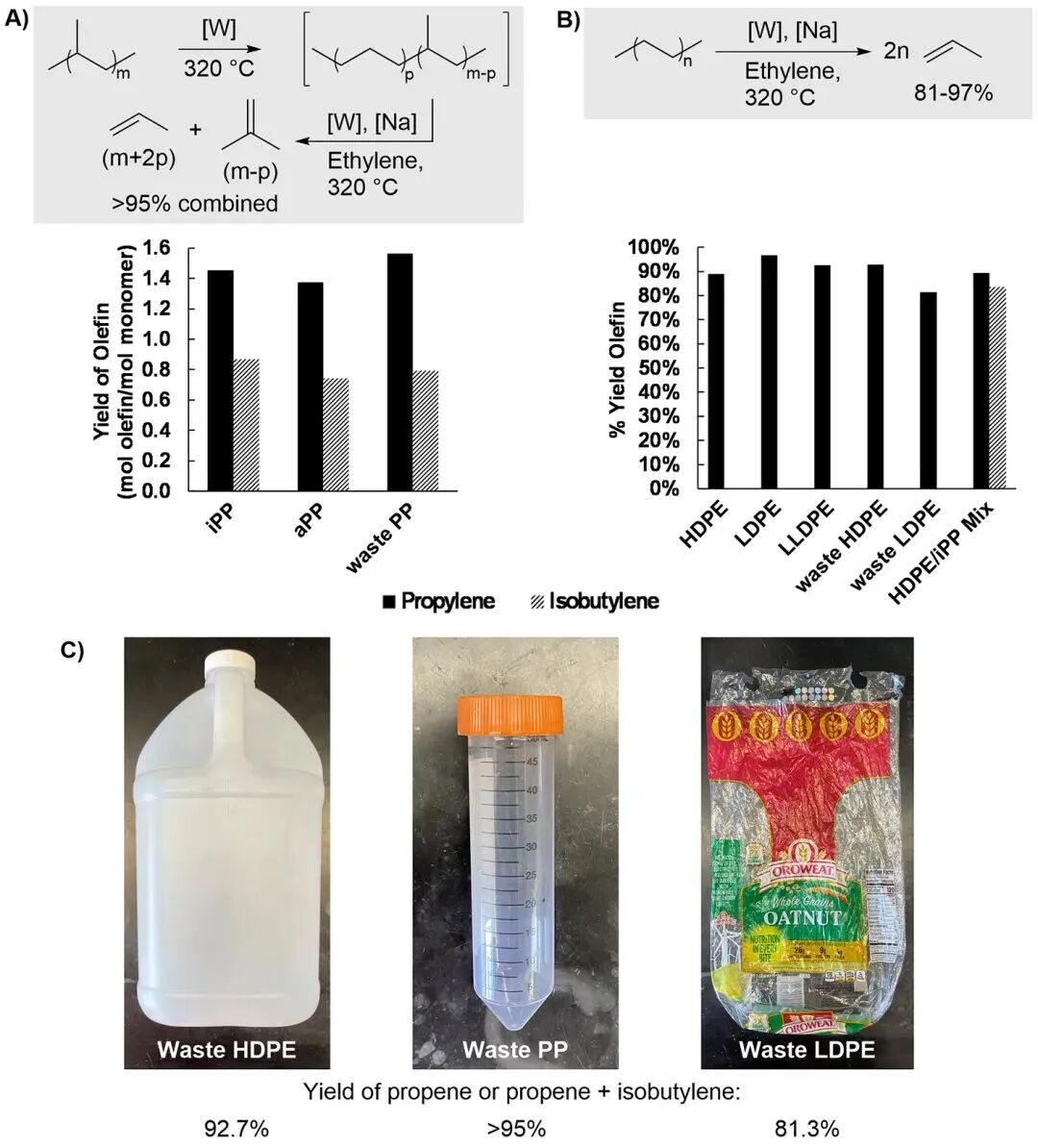
Figure 3 Tandem catalytic cracking of polyolefins and isomerization-ethanol decomposition (CIE) experiments
Post-consumer waste CIE
The author tested the effect of this catalytic system on the CIE reaction of post-consumer waste (Figure 3C). Pre-treated waste HDPE and PP samples produced products with a 93% yield and a propylene to isobutylene ratio of 2:1 in the reaction, with conversion rates comparable to commercial virgin samples. The reaction of mixed polymers (such as bread bags) also resulted in high yields, although the addition of contaminants like PVC or PET significantly reduced the yield of propylene. Isotope labeling experiments confirmed that propylene mainly originated from polyolefins rather than ethylene. Furthermore, the catalytic activity of the system remained stable over three cycles, and although the activity of Na/γ-Al2O3 gradually declined, it could be restored by adding fresh catalyst (Figure 4B).
Carry out a larger-scale reaction in a semi-batch reactor.
The author explored the feasibility of polyolefin CIE in a semi-batch reactor and found that by adjusting the ethylene flow rate and pressure, the yield of propylene and isobutylene can be significantly affected (Figure 4C). Under ambient pressure, the propylene yield of HDPE CIE is 26.9%, which reaches 84.5% at a pressure of 20 bar, consistent with batch experiment results. The CIE of PP exhibits high selectivity, with isobutylene accounting for 97% of the products. By gradually condensing the effluent, the author successfully separated a large amount of olefins from the continuous ethylene flow and large-scale PE reaction, demonstrating the industrial potential of this process (Figure 4D). Additionally, the author generated a large amount of advanced olefins, indicating that by optimizing reaction conditions, it is possible to flexibly produce mixtures of light or advanced olefins.
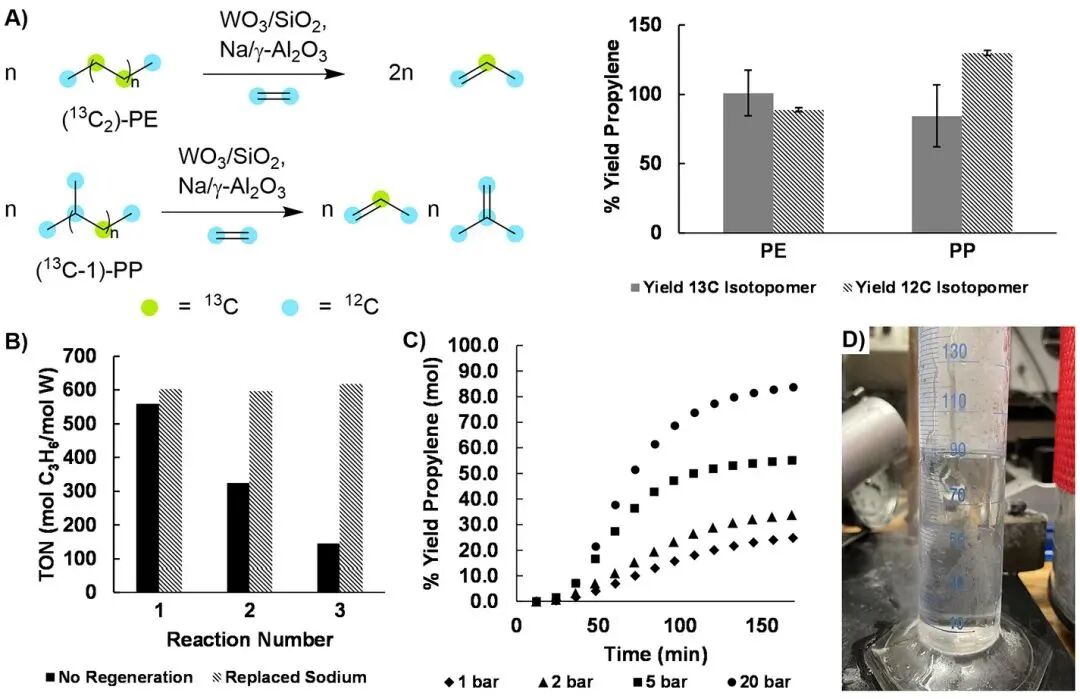
Figure 4 Mechanism, catalyst reuse, and scale-up
The results of this work demonstrate the feasibility of using inexpensive multiphase catalysts, either alone or in mixtures, to deconstruct PE and PP, the two largest-volume plastics, into products that serve as raw materials for the chemical industry. These catalysts can be recovered and used in semi-finished products. Although a robust techno-economic assessment is required to determine the economic viability of conducting this reaction on a commercial scale, the authors hope that the work described in this study will lead to practical methods for reclaiming carbon from PE and PP as propylene and isobutylene, which can be used to produce new polymers. By doing so, the demand for producing these essential commodity chemicals from fossil carbon sources, such as oil and natural gas, and the associated greenhouse gas emissions can be significantly reduced.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






