Starting from May 1, there will be a significant adjustment in the prices of consumables.
01
The era of "prices no lower than three provinces' prices" has ended.
Scrutinize abnormal high and low prices
On March 17th, the Qinghai Provincial Medical Insurance Bureau released a "Notice on Regulating the Direct Listing Prices and Procurement Requirements of Medical Consumables and In Vitro Diagnostic Reagents," adjusting the direct listing prices and procurement requirements for medical consumables and in vitro diagnostic reagents.

Starting from May 1st, for medical consumables and in vitro diagnostic reagents that have been listed on the network, products with the national lowest procurement prices of three provincial levels will only retain their lowest listed prices nationwide. Products with only one provincial listed price or declared price will retain their listed prices.
Relevant enterprises should proactively monitor the standardized adjustment of their挂牌价格 on the Qinghai Province Procurement Platform. If the national minimum挂牌价格 (including actual transaction prices) is lowered in other provinces, they should actively initiate price linkage and submit a price adjustment application to the Provincial Drug Procurement Center within 30 days. The Provincial Drug Procurement Center should promptly adjust the挂牌价格 and implement dynamic management.
Production enterprises should strictly adhere to the principle of honesty and integrity, and not exceed the national minimum purchase price (including transaction price) or the enterprise's reported price. The enterprises should not submit the national minimum three provinces provincial procurement price. The medical equipment and supplies products shall be submitted to the national medical insurance bureau through a unified portal in one step, and the medical equipment and supplies products shall be verified by one province.
Public medical institutions shall refer to the listed prices reported by enterprises for online bidding and negotiate procurement prices with enterprises, which shall not exceed the listed prices.
Prior to this notice, Qinghai Province had, through consultations with online market entities, urged timely updates and confirmation of online prices, resulting in the disposal of 3747 abnormal online pricing products across 10 batches, including 37 pharmaceutical products, 3586 medical consumables, and 124 diagnostic reagents, with the maximum reduction of 95.67% and an average reduction of 34.93%.
Also effective May 1st, the Hunan Provincial Medical Insurance Bureau released the "Notice on Establishing a Monitoring and Disposal Mechanism for Medical Price Information in Hunan Province." The document emphasizes monitoring information on the following: regions and medical institutions where medical expenses have increased excessively, grown too quickly, have unreasonable structures, or where residents' medical burdens have significantly increased; unfair high prices, discriminatory high prices, and malicious low prices of drugs and medical consumables. The monitoring of price information will be used to strengthen the self-discipline of medical institutions and guide the public towards rational medical treatment and medication purchases.
The specific monitoring content is as follows:

The listed prices of consumables are also within the monitoring scope. The document requires that medical security departments at all levels regularly collect the listed prices of drugs and medical consumables from the Hunan Provincial Medical Procurement Platform, as well as the actual transaction price information generated from trading orders.
In this price monitoring scope in Hunan, not only high-priced products but also abnormally low-priced products will be monitored. The time, location, price difference, and frequency of unfair high prices, discriminatory high prices, and malicious low prices caused by market恶性competition will be monitored and analyzed. The analysis results will serve as important leads for identifying and rectifying issues such as inflated listed prices, drug diversion, and fake drugs.
Except for Qinghai and Hunan, provinces such as Jiangsu, Heilongjiang, Inner Mongolia, Shaanxi, Hebei, Gansu, and Shandong have also successively launched governance work related to the pricing of consumables. Enterprises that fail to adjust their products in accordance with the requirements in a timely manner will face penalties such as removal from the network and credit score deductions.
02
Government Intervention
Deepening and Unification of Consumables Online Listing Reform
The main focus of the work in the medical insurance field this year is the "Efficient Implementation of One Thing" in the medical insurance sector of the 2025 annual first batch of key tasks issued by the State Administration of Medical Insurance Office in February. The State Administration of Medical Insurance Office issued the "Medical Insurance Sector 'Efficient Implementation of One Thing' 2025 Annual First Batch Key Tasks Clearing List", requiring medical products to be registered nationwide through the unified portal of the provincial or multiple provincial websites. Companies can submit medical products and prices through the unified portal of one province or multiple provinces, submitting the data once, and verifying the information nationwide.
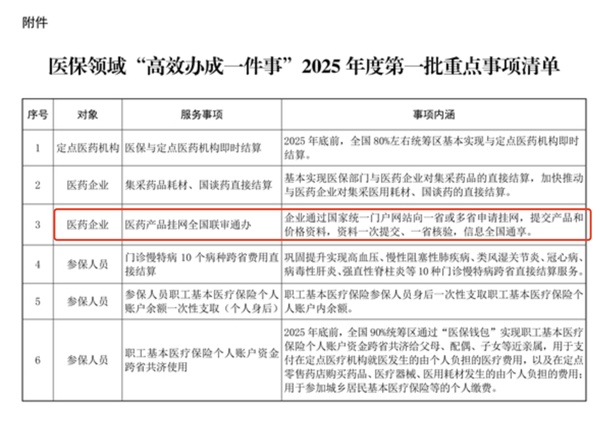
With the promotion of national joint review and handling, the process for enterprises to register online has been significantly simplified, allowing for a "one-step" completion of the registration work. At the same time, price supervision has become more transparent and timely, further reducing the space for false reporting.
Local price data sharing is also being promoted. In October last year, the Shaanxi Provincial Public Resources Trading Center organized a price comparison of drugs and medical consumables listed online. The comparison was based on the online listing data from the Shaanxi platform and compared with the platforms of Guangxi, Shandong, and Anhui provinces. For some products found not to meet the requirement of linking to the lowest listed price from other provinces, their listing qualifications were revoked, and no applications for listing that product would be accepted for one year.
While price linkage for挂网 (public tender) is implemented, the credit evaluation and punishment mechanism will be strengthened. Actions such as false declaration and untimely delivery will be subject to credit scoring systems, affecting companies' qualifications to participate in volume-based procurement.
Furthermore, the regulatory blind spots in the penalty mechanism have been further eliminated. Hunan has now stipulated the handling of abnormal price information for medicines and medical consumables. By referring to the risk management measures and relevant guidelines of the national medical security department, measures such as price verification, written inquiries, cost investigations, and price interviews will be taken to urge enterprises to proactively standardize their pricing behavior; for those who refuse to rectify without valid reasons or fail to rectify adequately, public inquiries will be conducted to require companies to publicly explain their specific pricing situations; for those who still refuse to rectify after public inquiries, credit evaluation measures will be imposed according to regulations; for enterprises reporting production halts and supply suspensions hoping to avoid price rectification, penetration to the marketing authorization holders will be made to prevent evasion of regulation through related party transactions.
The government's supervision of procurement also gradually extended to the online purchase market, as for example the Shanghai Medical Insurance Bureau issued a notice urging local medical institutions to actively push for self-service price negotiations for goods sold online through the "Sun Yang Hang" price regulation project, and for enterprises in the South of Shanghai to voluntarily participate in self-service price negotiations with designated medical institutions in the South. To this end, local medical institutions which had been authorized to buy the drugs and non-separate use medical consumables via online purchases would be granted bonus prizes of remaining funds, every year by March in accordance with the self-service price negotiations results, for every year's remaining funds the same was paid out.
With the improvement of price supervision regulatory mechanisms, cross-provincial price data sharing and the promotion of the "national unified examination and approval" mode, regional differences in consumable prices will be gradually eliminated, and a unified national minimum price system will be accelerated.
Attached:


【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

Key Players: The 10 Most Critical Publicly Listed Companies in Solid-State Battery Raw Materials
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Clariant's CATOFIN™ Catalyst and CLARITY™ Platform Drive Dual-Engine Performance
-

List Released! Mexico Announces 50% Tariff On 1,371 China Product Categories






