Myrtus announced the clinical trial results of Wrapsody CIE for establishing arteriovenous access.
Merit Medical announced that the six-month results of the Wrapsody Arteriovenous Access Efficacy Trial (WAVE Trial), randomized group, have been confirmed for publication in the April issue of Kidney International, while the twelve-month results of the trial will be officially presented at the 50th Scientific Session of the Society of Interventional Radiology (March 29 to April 2, Nashville, USA).
Wrapsody CIE received premarket approval (PMA) from the U.S. FDA in December 2024 and launched commercialization in the U.S. in January 2025. The device had previously obtained CE certification in the European Union and was launched in Brazil.
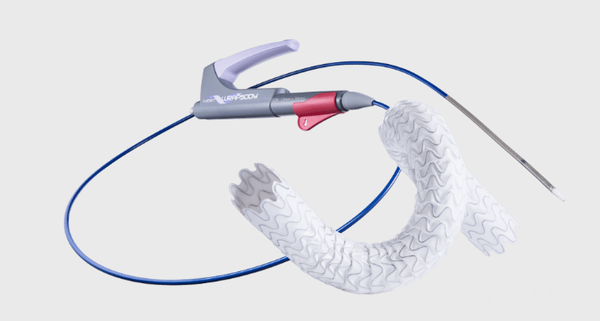
1. Background and Technology
Patients undergoing hemodialysis need to establish an arteriovenous fistula (AVF) to achieve a long-term vascular access. However, AVF and surrounding vascular stenosis can interfere with dialysis treatment and even lead to life-threatening consequences. The WRAPSODY™ Cell-Impermeable Endoprosthesis (CIE) is designed to assist clinicians in restoring vascular access function when stenosis occurs in the venous outflow tract of dialysis patients.
Inner layer: The innermost layer of WRAPSODY is made of a biocompatible novel spun polytetrafluoroethylene (PTFE) layer, which reduces fibrin deposition and thrombus formation without the need for coatings, chemicals, or drugs.
Middle layer: The impermeable graft layer, WRAPSODY uses an impermeable intermediate graft layer design that prevents transwall cell migration without the need for drug binding.
Outer layer: The outer layer of WRAPSODY is made of standard biocompatible expanded polytetrafluoroethylene (ePTFE) material, which promotes necessary tissue ingrowth to prevent stent migration.
2. Experimental Design and Results
The WAVE trial is a multicenter, international investigational device exemption (IDE) trial aimed at evaluating the safety and efficacy of the Wrapsody CIE device over a two-year period. In the randomized portion, 245 hemodialysis patients with AVF venous outflow tract stenosis were randomly assigned to receive Wrapsody CIE treatment (n=122) or standard percutaneous transluminal angioplasty (PTA, n=123).
-
Efficacy endpoints: the proportion of patients with primary patency of target lesions who do not require intervention due to clinically driven revascularization or thrombosis formation; primary patency of access, defined as the proportion of patients with no loss of vascular access at any site from the initial treatment until the need for reintervention or abandonment of the vascular access.
-
Safety endpoint: The primary safety definition is the proportion of patients experiencing reintervention, hospitalization, or death due to adverse events affecting vascular access or venous outflow tract (excluding target lesion revascularization or thrombosis).
-
Six-month results: The primary patency rate of target lesions in the Wrapsody CIE group was significantly higher than that in the PTA group (89.8% vs. 62.8%, p<0.0001); the primary patency rate of the access site was also significantly higher (72.6% vs. 57.9%, p=0.015); there were no significant differences in safety between the two groups.
-
12-month results: The primary patency rate of target lesions in the Wrapsody CIE group remains significantly higher than that in the PTA group (70.1% vs. 41.6%, p<0.0001); the primary patency rate of the access site continues to lead (58.1% vs. 34.4%, p=0.0003).
3. Expert Opinions
Dheeraj K Rajan, a researcher from the WAVE trial at the University of Toronto, stated: "The positive one-year results of Wrapsody CIE fill an important knowledge gap regarding the long-term durability of devices. It is encouraging that we now have a new device that can help extend the functional use of patients' vascular access."
Fred P Lampropoulos, Chairman and CEO of Merit Medical, said: "We are pleased to continue the outstanding performance of Wrapsody CIE. Merit is committed to improving patient care and providing clinicians with the data support they need for evidence-based decision making."
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






