Just now! Another key Philips product has completely exited the market
Philips spent 2.6 billion on the "first and only," which is completely exiting the market in less than 5 years!
01
Causing injuries to 20 people!
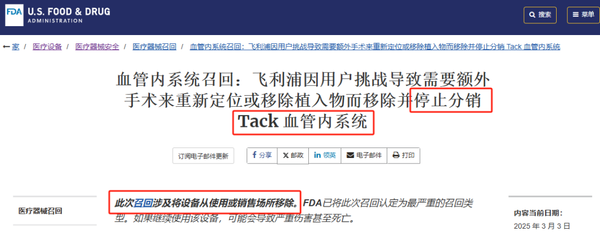
A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack endovascular system, with the recall level being the most severe, Class I, indicating that the product series may cause serious injury or death.
Due to serious safety concerns, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it.
Philips Spent 2.6 Billion on the "First and Only," Completely Exited the Market in Less Than 5 Years! 01 Causing Injuries to 20 People! A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack Endovascular System, with the recall level being the most severe, Class I, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will completely exit the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Philips Spent 2.6 Billion on the "First and Only," Completely Exited the Market in Less Than 5 Years! 01 Caused Injuries to 20 People! A few hours ago, the FDA officially announced that Philips is recalling its Tack Endovascular System, with the recall level being the most severe, Level I, indicating that the product series may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Philips Spent 2.6 Billion on the "First and Only," and It's Completely Exited the Market in Less Than 5 Years! 01 Caused Injuries to 20 People! A few hours ago, the FDA officially announced that Philips is recalling its Tack Endovascular System, with the recall classified as the most serious level, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Philips spent 2.6 billion on the "first and only," and it's completely exiting the market in less than 5 years! 01 Causing injuries to 20 people! A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack endovascular system, with the recall level being the most severe, Level I, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Philips has spent 2.6 billion on the "first and only," but it completely exited the market in less than five years! 01 Causing injuries to 20 people! A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack Endovascular System, with the recall level being the most severe, Class I, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Philips spent 2.6 billion to acquire the "first and only," which is completely exiting the market in less than 5 years! 01 Causing injuries to 20 people! A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack Endovascular System, with the recall level being the most severe, Level I, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Image source: FDA official website Philips spent 2.6 billion to acquire the "first and only," but it completely exited the market in less than 5 years! 01 Causing injuries to 20 people! A few hours ago, the FDA officially issued a notice that Philips is recalling its Tack endovascular system, with the recall level being the most severe, Class I, indicating that the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Image source: FDA official website The official notice states that the use of the affectedSure, please provide the content that needs to be translated. Philips Spent 2.6 Billion on the "First and Only," Exits the Market Completely in Less Than 5 Years! 01 Causing Injuries to 20 People! A few hours ago, the FDA officially announced that Philips is recalling its Tack Endovascular System, with the recall level being the most serious, Class I, which means the series of products may cause serious injury or death. Due to serious safety hazards, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Image Source: FDA Official Website The official notice states that using the affected product may result in serious adverse health consequences, including short-term risks such as partial or complete obstructionSure, please provide the content that needs to be translated. Philips Spent 2.6 Billion on the "First and Only," but It's Completely Exiting the Market in Less Than 5 Years! 01 Caused Injuries to 20 People! A few hours ago, the FDA officially announced that Philips is recalling its Tack Endovascular System, with the recall classified as the most serious Class I, meaning the product series may cause serious injury or death. Due to serious safety concerns, this product will be completely withdrawn from the market. Philips urges interventional cardiologists to immediately stop using it and remove it. Image Source: FDA Official Website The official notice states that using the affected product may result in serious adverse health consequences, including short-term risks such as partial or complete blockage of blood flow (occlusion), holes or tears in the inner wall of the artery (dissection) and penetration through the entireSure, please provide the content that needs to be translated. Philips Spent 2.6 Billion on the "First and Only," but It's Completely Exited the Market in Less Than 5 Years!