In-Depth Analysis: The Scientific Logic and Industrial Value Behind Chemical Recycling of Waste Plastics
Chemical recycling of plastic waste has become a key approach to addressing "white pollution" and achieving resource recycling. It can convert waste plastics into valuable products such as base oil, combustible gas, and carbon black, effectively reducing dependence on virgin resources and minimizing environmental pollution. This is not a pipe dream, but a scientific transformation based on the molecular characteristics of plastics.
The "Plasticity" of Plastic Molecules: Innate Conditions for Recycling
Common plastics in our daily lives, such as polyethylene (PE), polypropylene (PP), and polystyrene (PS), are all polymer compounds composed of carbon and hydrogen elements. Their molecules consist of long chains of carbon and hydrogen atoms, and different plastics vary in the length, structure, and bonding patterns of these chains.
For example, polyethylene is composed of a large number of ethylene monomers (C₂H₄) connected by covalent bonds to form a linear long chain with a relatively simple and regular structure; polypropylene is polymerized from propylene monomers (C₃H₆), with methyl side chains attached to the molecular chain. This hydrocarbon-based molecular structure determines the relationship between plastics and petroleum. It should be noted that petroleum can be processed to obtain various hydrocarbons such as gasoline, diesel, lubricants, etc., and plastics themselves are products of petrochemical processes. Therefore, essentially, waste plastics have the potential to be converted into products similar to petroleum derivatives.
This hydrocarbon property makes the chemical recycling of waste plastics possible. Through specific chemical methods, we can break the covalent bonds in plastic molecules, decomposing the long hydrocarbon chains into fragments of different lengths. These fragments, after further processing, can be converted into products such as base oil, combustible gas, and carbon black.
The core process of chemical recycling: "deconstruction and reorganization" of molecular chains
The chemical recycling of waste plastics is a complex and precise process, with its core being the "decomposition" and "reorganization" of plastic molecular chains. This process mainly includes steps such as pretreatment, cracking, and separation and purification.
In the pretreatment stage, the collected waste plastics need to be sorted and crushed. Different types of plastics have varying chemical properties and recycling processes; sorting can improve recycling efficiency and product quality. Crushing breaks large pieces of plastic into smaller ones, increasing the contact area with the reaction medium, which is beneficial for the reaction process.
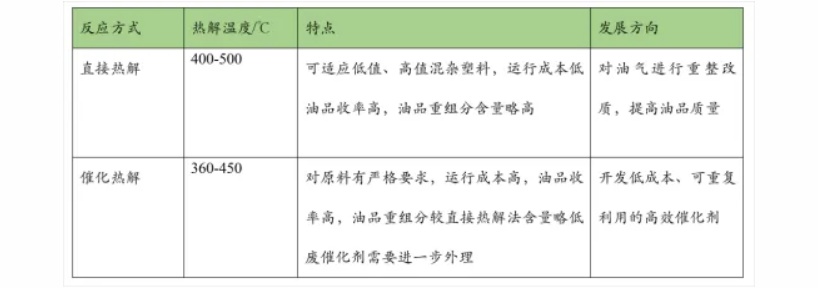
Pyrolysis is a key step in chemical recycling. It is the process in which plastic molecular chains break under the action of high temperature, high pressure, or catalysts. According to different reaction conditions, pyrolysis can be divided into thermal pyrolysis and catalytic pyrolysis. Thermal pyrolysis mainly relies on high temperature to break molecular chains, usually between 300-900°C; catalytic pyrolysis, on the other hand, lowers the activation energy of the reaction under the action of catalysts, allowing molecular chains to break at relatively lower temperatures while also improving the selectivity of target products.
During the cracking process, plastic molecular chains break according to certain patterns. Long-chain molecules first break into shorter fragments, which can further break down into even smaller molecules. Different reaction conditions affect the degree of chain scission and the distribution of products. For example, at lower temperatures (400-500°C) and with suitable catalysts, the formation of longer hydrocarbon chain fragments is favored. After separation and purification, these fragments can be used as base oils. When the temperature rises above 600°C, the molecular chains break more thoroughly, resulting in more short-chain hydrocarbons such as methane, ethane, and propane, which are the main components of combustible gases. In a high-temperature, oxygen-deficient environment, some plastic molecules undergo carbonization reactions, producing carbon black.
The separation and purification stage involves separating the mixture produced by cracking according to different properties such as boiling point and density, obtaining base oil, combustible gas, and carbon black with higher purity. This stage requires the use of various separation techniques such as distillation, adsorption, and filtration to ensure that the product quality meets the relevant standards.
Plastic Recycling: Different Types, Distinct Characteristics
Different types of plastics exhibit different characteristics in the chemical recycling process due to differences in their molecular structures and chemical properties.
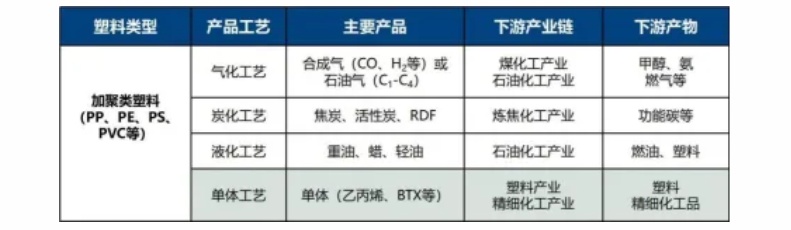
Polyethylene (PE) is one of the easiest plastics to chemically recycle. Its molecular structure is simple, with a reasonable carbon-to-hydrogen ratio, making it prone to producing a larger amount of liquid products, namely base oils, during the cracking process. Studies show that 1 ton of waste polyethylene plastic can yield 600-700 liters of base oil through appropriate processes. The performance of these base oils is comparable to traditional petroleum-based base oils and can be widely used in fields such as lubricants and hydraulic oils.
The chemical recycling of polypropylene (PP) also holds high value. During pyrolysis, it generates a significant amount of propylene and other olefin compounds, which can be used not only as combustible gases but also as chemical raw materials for producing new plastics. In addition, the liquid products produced from the pyrolysis of polypropylene can be processed and used as base oil.
The molecular structure of polystyrene (PS) contains benzene rings, making it prone to generating styrene monomer during the cracking process, which is an important raw material for the production of polystyrene. At the same time, polystyrene can also produce a certain amount of combustible gas and carbon black through cracking.
Chemical recycling of polyvinyl chloride (PVC) is relatively complex because its molecules contain chlorine elements. During the pyrolysis process, chlorine is released in the form of hydrogen chloride, which can cause equipment corrosion and environmental pollution. Therefore, in the chemical recycling of PVC, dechlorination treatment is required first, which increases the cost and difficulty of recycling. However, with continuous technological advancements, significant progress has been made in the chemical recycling of PVC, with improved dechlorination efficiency, making its recycling possible.
Chemical recycling of waste plastics is a technology of significant importance. It not only addresses the issue of "white pollution" but also enables resource recycling, offering notable environmental and economic benefits. With continuous technological advancements and policy support, it is believed that in the near future, chemical recycling of waste plastics will become a major method of plastic recycling, making an important contribution to sustainable development.
Source: Zhejiang Guosu Environmental Protection Group Co., Ltd.【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics






