From Familiar Plasticizers to Unveiling the Mysterious Polymer Free Volume
When selecting materials or developing flexible plastic formulations, engineers often encounter a mysterious yet crucial component: the plasticizer. It is often defined as an "additive that imparts flexibility to plastics," but how do plasticizers work? Why does the plastic become softer and more pliable after they are added, and why does the glass transition temperature (Tg) even decrease? All of this can actually be explained by an important concept in polymer physics: free volume.
What is "free volume"? Explain with an example from daily life.

Imagine you stack a pile of mahjong tiles tightly together, with almost no gaps between them. This is like the tight arrangement of rigid plastic chains. Once you sprinkle some small balls (like marbles) in between, the mahjong tiles get "pried open" and gain some room to move. These small balls are like plasticizer molecules. They insert themselves between the polymer chains, increasing the movable space between the chain segments, which is referred to as the "free volume."
In terms of physical meaning, free volume refers to the space in polymer materials that is available for segment movement after excluding the space occupied by polymer chains. The larger the free volume, the easier it is for the segments to move, and the softer and more easily deformable the material becomes.
Plasticizers increase free volume how?
The essence of the plasticizing effect of plasticizers is:
By introducing foreign molecules with low molecular weight and low Tg, these molecules insert themselves between polymer chains, disrupting the original interchain interactions and increasing the free volume of the material. This results in a decrease in the glass transition temperature, making the material soft and usable at room temperature.
Specifically, the way plasticizers increase free volume includes the following four steps, with the first three steps being indispensable (the core of the mechanism):
1. Reduce the attraction between chain segments - decrease interchain hydrogen bonds or van der Waals forces.Dipole-dipole interaction;
2. Physical separation of chain segments - Plasticizer molecules insert themselves, acting as "separators."
3. Enhance molecular mobility — especially increasing the freedom of the main chain, side chain ends, and other such parts.
4. Prevent recrystallization or aggregation — Keeping the chain segments in a disordered state helps to enhance the flexibility of the material.
Misconception: Many people mistakenly believe that BDP (a liquid flame retardant for flame-retardant PC/ABS) is a "plasticizer" and should make the material softer. However, in practical applications, BDP often increases the rigidity (modulus) of PC/ABS, and even makes it more brittle and prone to cracking.
The fundamental reason:BDP has high structural rigidity and strong polarity, and is highly compatible with PC/ABS.
BDP is a bisphenol A-based phosphate ester containing two bulky rigid aromatic rings.
It has the same origin structure as PC (polycarbonate) itself (also derived from bisphenol A).
At the molecular level, there are strong polar interactions between PC molecules (such as π–π stacking, hydrogen bonds, etc.).
The result is: Unlike traditional plasticizers, the BDP molecule does not increase the free volume, but may instead reduce segmental mobility.
Further: Why are some plasticizers "good" and others "bad"?
Although in theory many organic small molecules can be incorporated into polymers, to become a high-quality plasticizer, the following requirements must also be met:
Good compatibility with polymers (similar solubility parameter δ).
Molecular structure provides low glass transition temperature.
Appropriate polarity to facilitate interaction with polar polymers (such as PVC);
Low volatility to reduce migration and fogging.
Stable and reversible within the applicable temperature range.
This explains why phthalates (such as DOP, DINP) and fatty acid esters (such as DOA, DINA) are widely used in flexible PVC, while other low-polarity molecules have difficulty achieving similar effects.
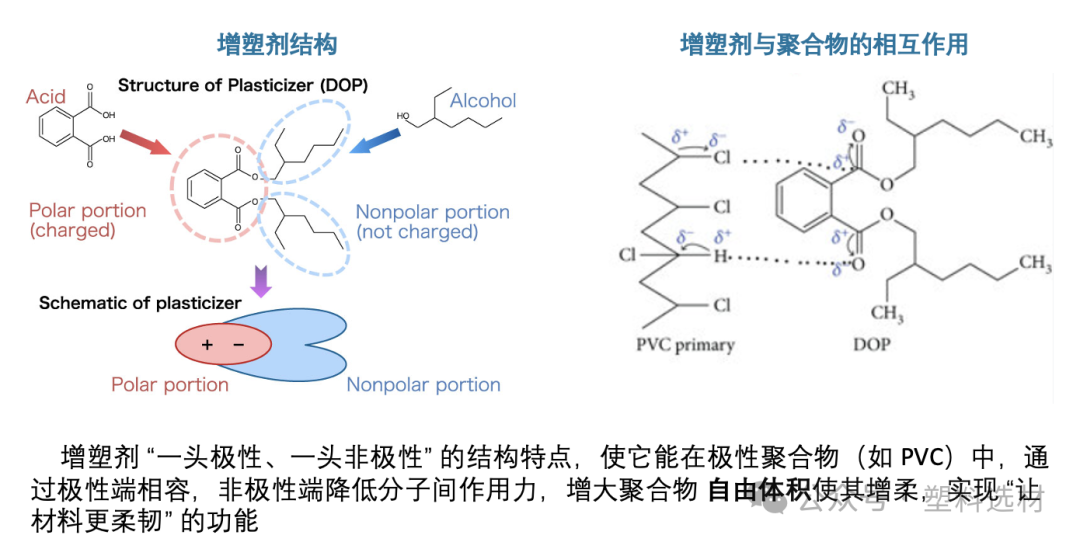
Too much plasticizer makes it harder instead?—The "anti-plasticization" phenomenon
Interestingly, when the amount of plasticizer is very low, it may actually make the material harder! This is known as the phenomenon of "antiplasticization."
The reason is that a small amount of plasticizer molecules preferentially bind with the polymer, causing the chain segments to arrange more regularly and the crystallinity to increase, leading to a harder material. Only when the amount of plasticizer added exceeds a certain threshold does the free volume significantly increase, beginning to exhibit "true plasticizing" behavior.
Conclusion: Free Volume - The Scientific Underpinnings of Choosing the Right Plasticizer
Understanding the concept of "free volume" can help engineers scientifically select plasticizers from the structure-performance relationship to optimize the flexibility, processability, and final performance of polymers. In the context of increasingly stringent environmental regulations and the vigorous development of new non-toxic plasticizers, this concept deserves more attention.
In the future, materials research and development is not just about the accumulation of formula experience, but also the precise manipulation of structural control. Plasticizers are not merely small molecules that "make plastics a bit softer"; they are actually a key that unlocks the door to the "free movement" space between molecules.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






