Finally, someone has clearly explained the "plasticizer" of polymers!
What is the essence of plasticizers?
Let's start with the name. The so-called "plasticizer" is something that can make materials more "plastic." Essentially, it is a type of...Small molecules or oligomersIt can insert between the polymer chains, reducing the forces between them, allowing the segments to move more easily.
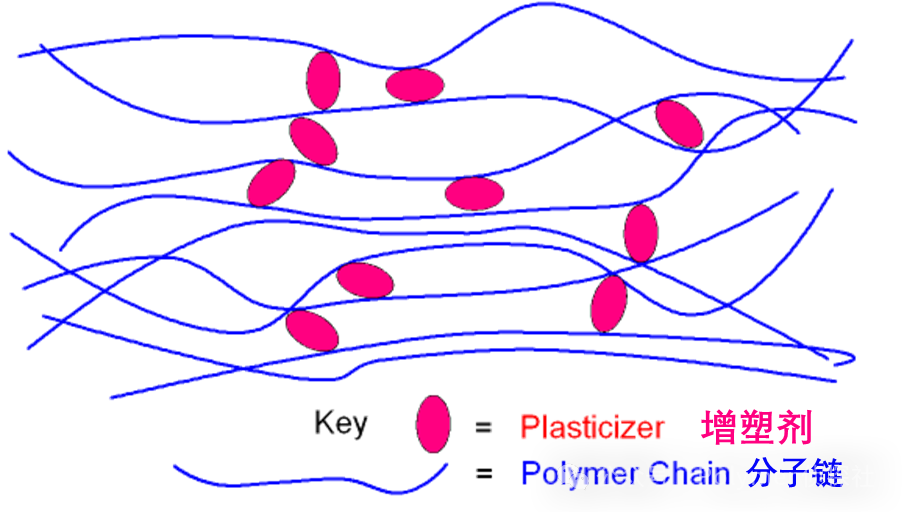
You can imagine it as a group of dancers (polymer chains) who are initially holding hands tightly, making their movements stiff and their dancing not flexible at all. Then, some "little lubricants" (plasticizers) come in quietly and slip between them, loosening their grip, and suddenly their dance movements become flexible.
From a molecular perspective, polymer chains are originally held together by hydrogen bonds, Van der Waals forces, and other interactions, making chain segments almost immobile at insufficient temperatures. The introduction of plasticizers acts like "inserting wedges."The binding energy between chain segments is weakened.The glass transition temperature (Tg) decreases, the segmental motion window opens earlier, and the material softens.
The essence of plasticizers can be summarized in one sentence:
It is a "molecular lubricant" that reduces the interaction force between chain segments and increases the freedom of movement of the chain segments.
What is the mechanism of action of plasticizers?
We often say that plasticizers make materials softer, but this statement is too general. In fact, from the molecular to the electronic level, its mechanism can be understood in at least three dimensions.
1. Thermodynamic Dimension: Free Volume Effect
The polymer chains are originally packed tightly, similar to a crowded subway during rush hour where people can barely move. When plasticizer molecules infiltrate, it's like "shoving in a few balloons," pushing the crowd apart. This creates more "gaps" (free volume) between the chain segments, significantly increasing the freedom of movement for the chains.
The direct result: the glass transition temperature Tg decreases, and the material becomes soft at a lower temperature.
Keywords: increase in free volume → decrease in activation energy barrier → decrease in Tg
2. Dimension of intermolecular forces: weakening the cohesive energy density
The hardness of polymers originates from intermolecular attractions such as hydrogen bonds, dipole-dipole interactions, and π-π stacking. These forces tightly bind the chains together.
When plasticizer molecules are introduced, on one hand, they "dilute" the attraction between polymer chains (similar to adding a bit of oil to glue), and on the other hand, they themselves can form some "weaker but flexible" interactions with the chains.
In PVC, the dipole of the –C=O (ester group) interacts with the –C–Cl segments, breaking the original pattern of "tight traction between Cl–Cl."
In polyamides, plasticizers weaken the hydrogen bond network, making the segments less rigid.
From the perspective of energy,Plasticizers reduce the cohesive energy density of the system.Make the chains "stick less tightly" together.
3. Electronic Behavior Dimension: Local Electron Cloud Perturbation
The attraction between polymer chains essentially comes from the distribution and interaction of electron clouds.
In polar chains (such as PVC, PA), the uneven distribution of electron clouds results in a large dipole moment and strong mutual attraction.
The introduction of polar groups of plasticizers (such as ester groups and hydroxyl groups) can couple with the dipoles of these segments, effectively "reshuffling" the electron distribution and weakening the original strong interactions.
In other words, the plasticizer acts as an "electronic buffer" between molecules: it interacts with the polymer chain segments using its own electron cloud, dispersing the originally tight dipole-dipole and hydrogen bond couplings.
This is also why different plasticizers have vastly different effects on different materials.Only when the electronic behavior matches can the plasticizer truly "insert and stay."
4. Dynamic Dimension: Shortened Segmental Relaxation Time
If you further examine the dynamic mechanical properties (DMA) of the material, you will find that after adding a plasticizer, the α relaxation peak (corresponding to the main chain segment motion) shifts to a lower temperature, and the peak broadens. What does this indicate?
The energy barrier for chain segment motion is reduced, and the material's...Relaxation time distributionBroader. In other words, plasticizers not only make the chain segments mobile but also enable chain segments of different lengths and in different environments to become active.
How to choose plasticizers for different material systems?
This is where many people encounter pitfalls. A plasticizer is not a "universal key"; it must match your material system. Otherwise, the effect may be insignificant, or it could lead to the major issues of "migration or precipitation."
Several Core Principles:
Compatibility First
A plasticizer must be compatible with your polymer chain. For example, PVC and phthalates are a good match because their polarities are compatible. However, if you use a non-polar hydrocarbon plasticizer (such as white oil) with PVC, it may not mix in at all and will eventually precipitate out.
Operating condition matching
Is your product used at room temperature or for long-term use at high temperatures? Is it used in air, or in oil, water, or solvent?
High temperature conditions: require low-volatility plasticizers (such as epoxy macromolecular plasticizers).
Contact with oil media: consider the risk of dissolution.
Medical and food contact: require low migration and low toxicity, such as citrate esters and bio-based plasticizers.
Physical Properties Requirements
Some systems need to be flexible but also resistant to low temperatures, so it is necessary to choose plasticizers with a low glass transition point and good cold resistance.
Some systems require flame retardancy, and functional plasticizers containing phosphorus and chlorine can be selected.
Processing requirements
Plasticizers also affect melt viscosity and processing flow. If your system is too viscous, adding a little low-molecular-weight plasticizer can make processing smoother. However, don't forget that it may also bring the risk of exudation.
Translate the above content into English and directly output the translation without any explanation.When choosing a plasticizer, you should look for one that "can enter, stay, and doesn't run around."
04 How to quickly determine if a plasticizer is suitable? (The most important point)
This is also the issue that everyone is most concerned about. Many people make samples immediately after a formula is released, only to find out from customer feedback that there is a problem with the plasticizer. In fact, it can be quickly verified in advance.
Several practical methods:
Accelerated migration testing
High temperature aging (e.g., 70°C, 1000 hours), check if the surface is sticky or exuding oil.
Soak in solvent, measure weight change, and observe how much migration occurs.
Vacuum oven, simulating low-pressure evaporation.
DSC Test
The more significant the decrease in Tg, the stronger the effect of the plasticizer added; if there is almost no change, it may indicate poor compatibility.
Infrared or NMR characterization
You can observe whether there are significant interaction peaks between the plasticizer and the matrix. If there is little interaction, the probability of future migration is high.
Rule of thumb
The most straightforward principle is "like dissolves like": choose polar plasticizers for polar systems and non-polar plasticizers for non-polar systems. Don't try to "force it," or there will be endless troubles.
In Conclusion
Many people think that plasticizers are minor components that can solve problems by simply adding any one of them. However, the reality is quite the opposite:The plasticizer is one of the key points for the system to remain stable in service.。
I have always told my group members:
Don't wait until the product fails and customers complain to ask "why did the plasticizer precipitate?"
At that time, you will realize that not only have you lost the trust of your clients, but more importantly, the effort you spent months developing has all been in vain.
So,It is essential to conduct accelerated evaluation and validation of plasticizers in the early stages.Don't be afraid of trouble; if you do it one day earlier, you'll have ten times less trouble.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






