Expanding Mainland China Workforce Fivefold, Medtech Giant Defies Market Downturn
In the trade war, more and more medical device giants are betting on both the U.S. and Chinese markets simultaneously.
Recently, Simon Michel, CEO of Ypsomed, a global leader in infusion, announced that they will establish a local production line for the U.S. market starting in 2027. He also revealed that the number of employees in China will increase to 500 over the next ten years.
It is understood that Ypsomed currently has approximately 2,000 employees worldwide engaged in the self-injection pen business, with three-quarters in Switzerland, about 400 in Germany, and 100 in China. This means that in the future, its workforce in China will be five times the current size.
01
Multiple favorable factors stimulate global expansion.
Ypsomed is a global leader in the development and manufacturing of self-injection and infusion devices, as well as an authoritative expert in diabetes care, holding a leading position in the global insulin pen market. Headquartered in Burgdorf, Switzerland, the group has established comprehensive production facilities, subsidiaries, and distribution networks worldwide. In 2024, the company celebrates its 40th anniversary.

Ypsomed is the preferred partner for numerous pharmaceutical and biotechnology companies, offering products such as injection pens, auto-injectors, and infusion pump devices for administering liquid medications. Through its mylife Diabetescare brand, Ypsomed provides a product portfolio directly to patients, pharmacies, and hospitals; meanwhile, leveraging the Ypsomed Delivery Systems brand, it conducts B2B business with major pharmaceutical companies.
Recently, the Trump administration imposed a 39% tariff on Switzerland, significantly higher than the 15% imposed on EU countries, making it the highest among all developed countries. Switzerland is home to many global healthcare giants, such as Medtronic, Novartis, Roche, and the world's largest hearing aid company, Sonova Group. The responses of these companies to the tariff war are of great concern.
In an interview, Simon Michel, CEO of Ypsomed, stated that currently, less than 10% of the company’s sales come from the US market, and the company does not manufacture in the US. Half of its products imported to the US come from Germany, and the other half from Switzerland. However, in the event of a second Trump term, we have decided to begin local production for the US market in the second half of 2027. In the medium term, the share of the US market is expected to rise to 20% of our sales.
He also revealed that the company has made it clear that the tariffs imposed by U.S. authorities will not be a reason for price reductions, and the additional import costs will be borne by customers. In addition, the company has decided to transfer the execution of U.S. orders from the Swiss company to its German subsidiary to avoid the 39% tariff.
By 2028, MedPace's investment in its US factory is expected to slightly exceed $300 million.
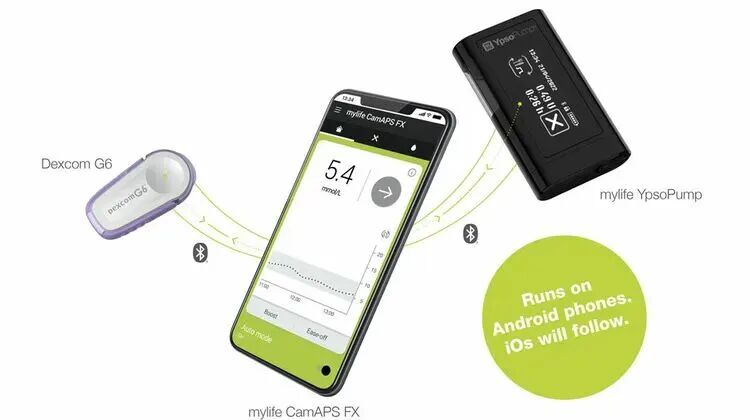
The medical infusion pump paired with CGM forms an intelligent automated insulin delivery (AID) system.
The incidence of chronic diseases such as diabetes and obesity is continuously rising, driving rapid growth in the injection device market. Known as a weight-loss miracle drug, GLP-1 has further fueled the growth of infusion devices. In the first half of 2025, the global GLP-1 drug market is expected to historically surpass PD-1/PD-L1, with sales exceeding $35 billion, and the annual market size is expected to surpass $70 billion.
GLP-1 drugs can be self-injected in liquid form using Yipaisen's injection pen. There is a target population of 1 billion obese individuals worldwide who are at risk for more than 200 related diseases. Benefiting from multiple positive market trends, as of March 31, 2025, Yipaisen's latest fiscal year comprehensive sales reached 749 million Swiss francs (approximately 6.73 billion RMB), representing a 38% increase compared to the previous year.
In the future, MedPage will expand its staff globally, and China is one of its key markets.
Simon Michel introduced that the company currently has about 2,000 employees working in the self-injection pen business, with three-quarters based in Switzerland and the rest overseas—around 400 in Germany and 100 in China. It is expected that in ten years, we will have about 2,000 employees in Switzerland, 1,000 employees in Germany, and 500 employees each in China and in the new factory in the United States.
Interestingly, Simon Michel also added that perhaps in the future, not all employees in Switzerland will be "human"—some roles, especially in research and development, intellectual property, and procurement, could be filled by virtual agents or artificial intelligence (AI). We are heavily investing in AI, implementing dozens of projects.
02
The first in the Asia-Pacific region to be established in Changzhou
After supplying products to China for over 15 years, Medpace has chosen to establish its first manufacturing base in China in Changzhou National Hi-Tech District, Jiangsu, marking an important step in its strategic layout in the Asia-Pacific region.
On June 26, 2025, Medtronic held a grand celebration to mark the official completion of its first exclusive production base in China. The new factory is located in the Changzhou High-tech Industrial Park, covering an area of 15,000 square meters, and will produce 100 million injection devices annually for the Chinese market. Notably, this base is Medtronic's first production facility established in the Asia-Pacific region.

According to the introduction, the company has invested nearly 150 million Swiss francs in the base and has reserved an additional 30,000 square meters of land for future expansion. The first batch of commercial products has also been successfully put into production.
Apart from Changzhou, Medipace is advancing its global production expansion on multiple fronts. In the United States, site evaluation for a new production base has entered the final stages, with the project expected to launch within the year. In Schwerin, Germany, construction of a second factory broke ground this spring. Meanwhile, in Switzerland, the production bases in Burgdorf and Solothurn are also undergoing continuous capacity optimization and expansion.
Currently, China has become the country with the largest number of diabetes patients in the world. According to statistics, in 2021, the number of adult diabetes patients in China reached 140 million, ranking first globally. It is estimated that this number will rise to 160 million by 2030 and to 170 million by 2045. The massive patient base provides a broad development space for the diabetes drug and medical device market.
03
Currently, the main players in the injection pen market are companies such as Ypsomed and SHL. According to data from the consulting firm MarketsandMarkets, the injection pen market is expected to grow at an annual rate of 7.9% from 2024 to 2030, reaching USD 74.1 billion. UBS Switzerland predicts that by 2029, sales of GLP-1 drugs will rise to USD 126 billion, reflecting the surge in chronic diseases.
In April of this year, Medtronic announced that it would sell its diabetes care business to TecMed for $517.7 million (approximately 420 million Swiss francs or 3.8 billion RMB). The company's CEO stated that the sale was due to the company's focus on seizing the significant opportunities in the subcutaneous self-injection market and expanding its global production capacity.
As an important partner of renowned multinational pharmaceutical companies such as Pfizer, Sanofi, Roche/Genentech, Eli Lilly, Merck, GlaxoSmithKline, and AstraZeneca, Medpace has built a technological moat in the contract manufacturing field through its portfolio of over 2,000 patents.
Domestic companies such as Hanerxi, Wanhai Medical, Chengji Biotech (Hanyu Pharmaceutical), Gangan Technology (Ganli Pharmaceutical), Delphi, Meihua Medical, Tonghua Dongbao, and Zhongshan Huifeng are gradually breaking the monopoly. With the restructuring of global supply chains and the impact of tariffs, whether there will be significant changes in the future market landscape remains to be seen. Medical Device Home will continue to monitor this development.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






