Approved CE! Abbott's First Balloon-shaped PFA Officially Launched
On March 27, 2025, Abbott (NYSE: ABT) announced that its Volt Pulsed Field Ablation (PFA) System has received CE Mark approval in Europe.
The company has begun collaborating with doctors who previously used the Volt system in Abbott's PFA clinical trials to conduct commercial PFA cases. Abbott plans to expand the system's promotion in the European market in the second half of 2025.
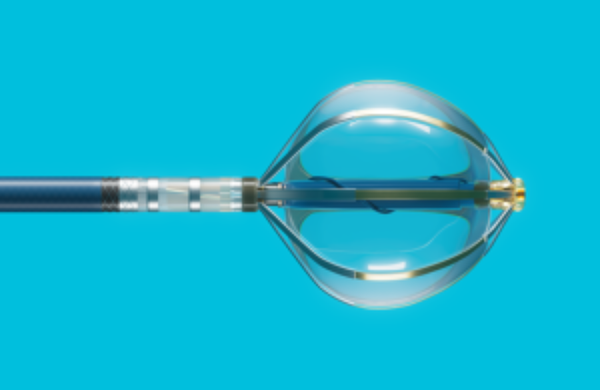
# Volt PFA System
PFA is a non-thermal ablation technique that delivers high-energy electrical pulses to targeted areas of heart tissue, disrupting abnormal electrical signals that cause arrhythmias.
Compared with traditional thermal ablation methods, PFA reduces the risk of damaging adjacent tissues, making it an attractive option for patients with complex cardiac anatomy or diseases.
Product Features
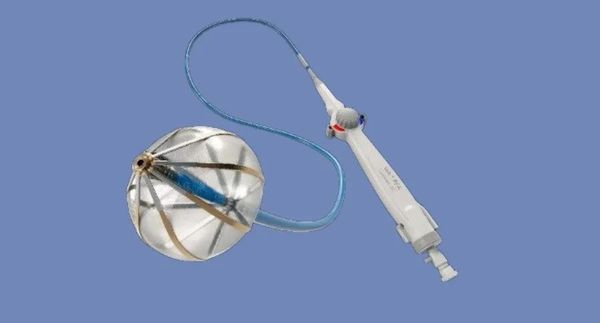
-
The distal end of the Volt PFA ablation catheter is a balloon, rather than other atypical shapes (such as ring-shaped or flower-shaped). The balloon is fitted with eight ablation band electrodes, allowing the catheter to closely adhere to the pulmonary vein vestibule, efficiently delivering energy directly to the target tissue and reducing the number of treatment applications required during the ablation process.
-
Patients who undergo the Volt PFA catheter (sensor-enhanced) minimally invasive ablation procedure can choose either mild sedation or general anesthesia according to the preference of the doctor and hospital.
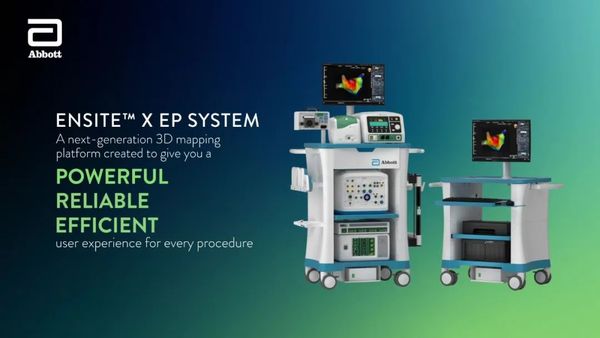
-
The Volt PFA system integrates with Abbott's EnSite X EP system, enabling mapping, pacing, and ablation with a single catheter, providing clearer visualization and more accurate navigation. It enhances visualization capabilities and real-time contact assessment to optimize catheter positioning. The EnSite X platform is suitable for all EP procedures, offering physicians performing PFA and other ablation procedures a "one-stop solution."
Dr. Christopher Piorkowski, Chief Medical Officer of Abbott's electrophysiology business, stated, "Clinical data also demonstrate that the tip design of the Volt catheter can achieve pulmonary vein isolation with just a single ablation, thereby improving patient outcomes."
Currently, Boston Scientific, Medtronic, and Johnson & Johnson have PFA products approved by the FDA, CE, and NMPA for market release. Other competing PFA systems on the market require multiple treatments when the catheter is positioned at different locations due to a lack of visualization or contact assessment.
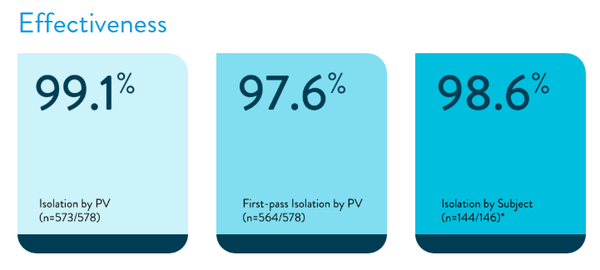
Abbott's Volt PFA system features a balloon-shaped design, achieving a pulmonary vein isolation (PVI) success rate of up to 99.1%, with significantly fewer energy releases required compared to competing PFA systems on the market. The launch of Volt PFA may drive the development of the entire PFA industry, and balloon-based PFA ablation technology is expected to become an important school in the evolution of PFA.
# Research data
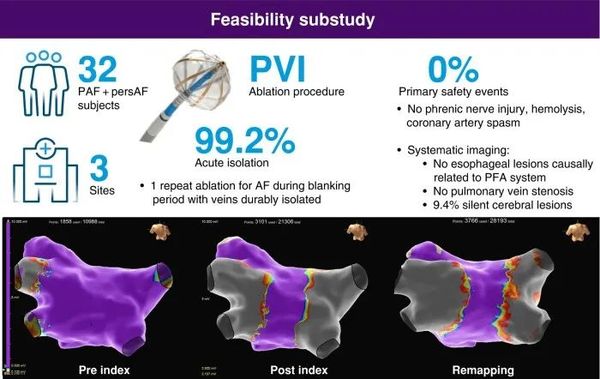
At the 30th International Atrial Fibrillation Symposium (AF Symposium 2025) held on January 17, 2025, Prof. Roland Tilz reported the acute results of the Volt CE Mark feasibility study.
The Volt CE Mark Feasibility Sub-Study aims to evaluate the safety and acute effectiveness of the Volt™ Pulsed Field Ablation System for the treatment of Atrial Fibrillation (AF).
-
Pulmonary vein isolation status: Among the 128 pulmonary veins treated, 127 achieved acute efficacy, accounting for 99.2%. In most pulmonary veins, PFA successfully achieved electrical isolation of the pulmonary veins, blocking the conduction of abnormal electrical signals, thereby helping to control atrial fibrillation.
-
At the subject level: Among 32 subjects, 31 achieved acute efficacy, accounting for 96.9%.
-
PFA application frequency: Each subject underwent an average of 23.8 ± 4.2 PFA applications, with each subject needing approximately 23.8 pulse field ablation operations on average.
-
Safety: No esophageal injuries have been found to be causally related to the Volt™ PFA system. In traditional thermal ablation treatments, the ablation energy may cause damage to the adjacent esophagus, leading to serious complications such as esophageal ulcers, esophageal stenosis, and even atrioesophageal fistulas.
# PFA Product Portfolio Progress
While obtaining CE certification, Abbott has also made regulatory and clinical progress in its other PFA product portfolio.
The company's Volt PFA system is also being evaluated in the VOLT-AF investigational device exemption (IDE) study, which completed enrollment of nearly 400 patients four months ahead of schedule. The 12-month follow-up for this study is expected to conclude later this year.


The financial report shows that Abbott's global sales in the fourth quarter of 2024 reached $11 billion, with an organic growth of 8.8% year-over-year. The adjusted diluted earnings per share for the fourth quarter were $1.34.
From the annual data, Abbott's global sales reached $42 billion in 2024, with a 7.1% organic growth year-over-year. The full-year sales achieved a 9.6% organic growth. The adjusted diluted earnings per share for the full year of 2024 were $4.67.
Looking ahead to 2025, Abbott is expected to achieve organic sales growth of 7.5% to 8.5% globally, with full-year adjusted diluted earnings per share ranging from $5.05 to $5.25.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






