Approved by FDA! Novel Guiding Catheter for Stroke
Recently, the Irish company Perfuze, which specializes in developing thrombectomy devices for stroke patients, announced that its new guide catheter Zipline has been approved by the FDA for market release, and it has completed a financing of 22 million euros (approximately 170 million RMB).
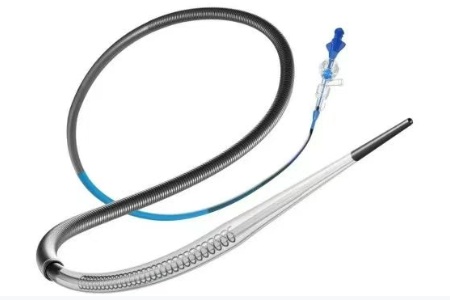
▲New Guide Catheter Zipline
Stroke is a common and serious cerebrovascular disease, which refers to the occlusion of cerebral blood supply arteries due to the formation of atherosclerotic plaques, plaque rupture, thrombosis, or embolism, leading to ischemic and hypoxic necrosis of brain tissue, thereby causing neurological dysfunction.
This disease is characterized by high incidence, high disability rate, and high mortality worldwide, bringing a heavy burden to patients and their families. According to statistics, one quarter of adults over the age of 25 globally will experience a stroke in their lifetime. In the treatment of stroke, time is crucial, and rapid restoration of blood flow to the brain is key to improving patient outcomes.
In recent years, with the continuous advancement of medical technology, the treatment methods for stroke have also been continuously developing. Endovascular intervention, as an effective treatment method, has gradually become the focus of clinical attention. Among these, the guide catheter plays a crucial role in endovascular intervention. It can provide a stable delivery channel for interventional devices, helping doctors to more accurately reach the lesion site, thereby improving the success rate and safety of the surgery.
Zipline can significantly improve the tracking and delivery performance of large inner diameter (070) and ultra-large inner diameter (088) aspiration catheters, simplifying the neurointerventional procedure and achieving faster and more efficient stroke treatment.
By enhancing support and operational flexibility, Zipline is committed to optimizing thrombus removal efficiency, thereby improving surgical success rates and patient outcomes. FDA approval marks a significant step forward in Perfuze's mission to simplify and improve stroke treatment and outcomes through technological innovation.
# Executive and Expert Evaluations
Perfuze Chairman Hooman Hakami commented: "The continued support from investors demonstrates confidence in our technology and vision. This round of funding will help us to conduct limited market promotion in the US and advance the implementation of revolutionary stroke treatment solutions at selected centers."
"Zipline's FDA approval demonstrates our commitment to developing world-leading stroke solutions. This authorization will strengthen our presence in the U.S. market and drive us to provide truly innovative, efficient, and user-friendly technologies that transform the landscape of stroke treatment."
"Zipline catheter represents an innovative technology that I believe will simplify stroke intervention, reduce costs, and accelerate reperfusion. Based on my initial experience, they can achieve rapid clot access and aspiration even in complex anatomies," said Dr. Jay Dolia, Assistant Professor of Neurology at Emory University School of Medicine.
Perfuze is an Irish company that currently focuses on the development of ultra-large bore thrombectomy technology, also known as the Millipede catheter. The founders of the company are Wayne Allen and Liam Mullins.
The company has undergone three rounds of financing, and the known specific information is as follows:
-
On January 29, 2019, the company announced that it had raised 3 million euros in a seed funding round, led by EarlyBirdCapital, with participation from MedFocus Fund and Enterprise Ireland. -
On February 9, 2022, the company announced that it had raised 22.5 million euros in Series A financing, led by Seroba Life Sciences and EQT Life Science, with participation from SV Health Investors, Medtech Convergence Fund, LSP Health Economics Fund, HBM-MedFocus, and EarlyBird. -
In March 2025, the company announced the completion of a 22 million euro financing. This round of financing was led by existing investors (Earlybird, EQT Life Sciences, Seroba, and SV Health, among others). The funds will support the commercial promotion of Perfuze's two marketed products, the guide catheter Zipline and the large-bore aspiration catheter Millipede, in the United States, and advance the clinical progress of its pipeline products.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






