Advantages of caprolactam method for producing hexamethylenediamine, with: Hexamethylenediamine Planned Capacity and Process Table
The caprolactam method (usually referring to the route of hydrogenating caprolactam to produce 6-aminocapronitrile, which is further hydrogenated to produce hexamethylenediamine) has the following significant advantages:

The advantages of the caprolactam method.
1. Shorter process, fewer steps:
Traditional Adipic Acid Method: Benzene -> Cyclohexane -> Cyclohexanol/Ketone -> Adipic Acid -> Adipic Acid Ammonium Salt -> Dehydration -> Adiponitrile -> Hydrogenation -> Hexamethylenediamine. This route is lengthy, involving multiple reaction units (oxidation, dehydration, hydrogenation), and the step of dehydration of adipic acid to produce adiponitrile has a relatively low yield (about 90-93%) with many by-products (such as cyclopentanone).
Caprolactam process: Cyclohexanone oxime -> (Beckmann rearrangement) -> Caprolactam -> (Ring-opening/hydrogenation dehydration) -> 6-Aminocapronitrile -> (Hydrogenation) -> Hexamethylenediamine. The core principle leverages the key intermediates in caprolactam production (cyclohexanone oxime or caprolactam itself), obtaining hexamethylenediamine through relatively more direct steps of ring-opening, dehydration (to form nitrile), and subsequent hydrogenation. This process involves significantly fewer steps compared to the adipic acid method, thus reducing intermediate stages.
High selectivity, fewer by-products.
Caprolactam hydrogenation to produce 6-aminocapronitrile (or similar intermediates) and the subsequent hydrogenation of 6-aminocapronitrile to produce hexamethylenediamine typically exhibit high selectivity.
Compared to the unavoidable by-products such as cyclopentanone in the dehydration process of adipic acid to produce adiponitrile (which affects yield and the cost of separation and purification), the caprolactam route generates fewer types and lower amounts of by-products, resulting in a relatively higher yield of the target product (theoretically up to 95% or more).
3. Synergy and Flexibility of Materials and Processes:
For chemical companies that already have large-scale caprolactam production facilities (such as BASF, DSM, Asahi Kasei, Sinopec, PetroChina, etc.), this route can directly utilize existing caprolactam capacity or intermediates (cyclohexanone oxime) to achieve shared facility use and raw material mutual supply.
This offers great production flexibility: companies can flexibly adjust the production ratio of caprolactam (used for nylon 6) and hexamethylenediamine (used for nylon 66) according to market demand, optimize product structure, and better respond to market fluctuations. This "one head, two tails" model is economically very attractive.
4. Potential environmental advantages (compared to the traditional adipic acid method):
Avoid the use of nitric acid: The core step in the traditional adipic acid production method is the nitric acid oxidation of cyclohexanol/ketone. This process generates a large amount of nitrous oxide (N₂O), a potent greenhouse gas, as well as nitrogen oxides (NOx) wastewater, which are both challenging and costly to treat environmentally.
Caprolactam production: In its initial stage (production of caprolactam), the process mainly employs either cyclohexane oxidation (yielding few by-products but with low yield) or cyclohexanone-oxime method (involving hydroxylamine synthesis, which presents some environmental challenges). However, it does not involve the nitric acid oxidation step, thus completely avoiding the generation of N₂O and nitric acid-related pollutants. If the production of caprolactam adopts a more environmentally friendly process (such as titanium-silicate molecular sieve catalyzed cyclohexanone ammoximation), the overall environmental advantage is even greater.
5. Energy consumption and operating conditions:
Although a detailed engineering calculation is required for a specific energy consumption comparison, the overall energy consumption is expected to be lower than the lengthy adipic acid method due to the shortened process and reduced reaction steps.
The conversion of caprolactam to 6-aminocapronitrile and the subsequent hydrogenation typically occur under relatively mild and controllable conditions of temperature and pressure.
Compared with the butadiene method
The butadiene process (such as INVISTA's ADN technology: butadiene -> hydrocyanation -> adiponitrile -> hydrogenation -> hexamethylenediamine) is currently the most advanced, cost-effective, and largest-scale production process for hexamethylenediamine. It has core advantages such as abundant raw material sources (C4 fractions), a relatively reasonable process flow, and significant economies of scale.
Compared to the butadiene method, the main disadvantage of the caprolactam method is:
Raw material cost sensitivity: The price of caprolactam is usually higher than that of butadiene, and its cost directly determines whether the route is economically competitive. When caprolactam prices are high, the competitiveness of the route decreases.
Scale effect: The butadiene process has a huge single-line capacity (such as INVISTA technology, which can reach hundreds of thousands of tons per year), resulting in extremely significant scale benefits. The caprolactam process usually relies on existing caprolactam facilities, and its scale may be limited by upstream factors.
The advantage of the caprolactam method over the butadiene method is:
The strategic value for existing caprolactam producers: For companies with large existing caprolactam production capacity that wish to enter the nylon 66 market, this route is the most effective way to quickly enter the market, revitalize assets, and achieve flexible production. It avoids the significant investment and technical barriers required for building new butadiene-based facilities, as the core technology for the butadiene method is monopolized by a few companies.
The stability of raw material supply: In certain regions or markets, the supply of butadiene may be tight or subject to significant price fluctuations, whereas the supply of caprolactam is relatively stable (especially for self-producing companies).
Avoid technology licensing fees: no need to pay expensive licensing fees for the butadiene method of producing adiponitrile.
Hexamethylenediamine Planned Capacity and Process Table
2025 China Hexamethylenediamine (HDA) Major Capacity Planning Summary Table
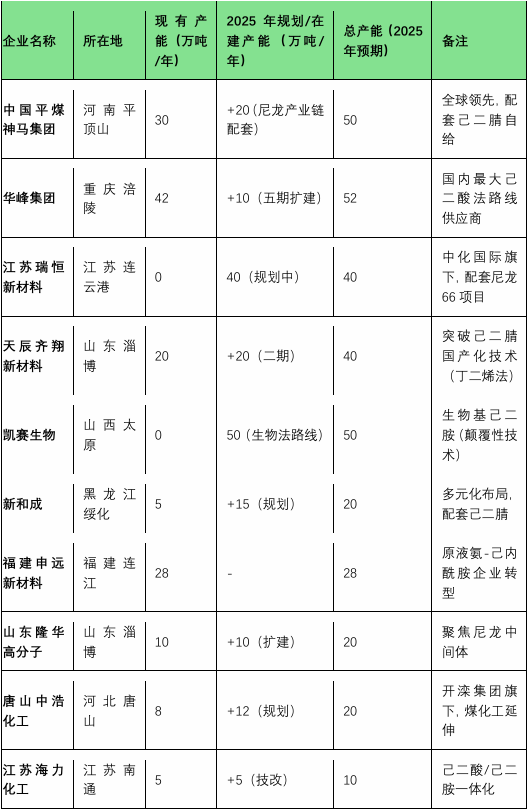
Data is based on corporate announcements, environmental assessment documents, and industry association reports (such as the China Chemical Fibers Association, Zhuo Chuang Information).
China Hexamethylene Diamine Production Capacity Process Classification Summary Table
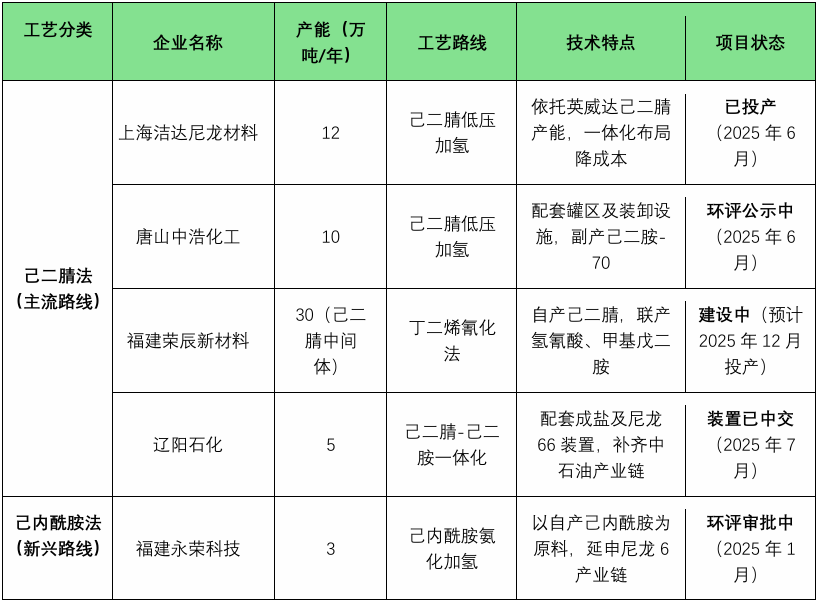
Advantages Summary
The core advantage of producing hexamethylenediamine via the caprolactam process is:
The process is significantly shortened, with fewer reaction steps.
High selectivity of the reaction, few by-products, and relatively high yield.
For large caprolactam producers, a high level of synergy between equipment and raw materials has been achieved, along with great production flexibility (switching between Nylon 6/66).
The traditional adipic acid method's nitric acid oxidation step is avoided, eliminating major sources of environmental pollutants such as N₂O, providing significant environmental advantages.
The economic competitiveness of this route is highly dependent on the market price of caprolactam and whether the company has a low-cost, large-scale caprolactam production base. For existing caprolactam giants, entering the hexamethylenediamine market is a strategic choice to optimize product portfolios and enhance asset utilization efficiency, offering significant economic and operational flexibility advantages. However, when facing the large-scale, low-cost butadiene method, its raw material cost disadvantage makes it difficult to become a new mainstream route, serving more as an important supplement and flexible production strategy for existing companies.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






