A Simple Guide to Understanding Heat Stabilizers
In the yellowed corners of outdoor billboards, in the brittle cracks of canopy supports, and on the fractured surfaces of furniture edge bands—all reveal the same material dilemma: the continuous degradation of PVC chains. As a technician who frequently adjusts PVC formulations, do you truly understand why stabilizers are not merely "additives," but the key cornerstone for the structural stability of PVC? How do they delay decay at the molecular level? And why do they suddenly become "powerless" at a certain point?
1. Why Must PVC Use Stabilizers? — A Specific Manifestation of Its "Innate Defects" from the Perspective of Molecular Structure
The molecular chain of PVC is formed by countless repetitions of "-CH₂-CHCl-", but about 1% to 5% of the structure has "inherent flaws": the root cause is deeply embedded in its chemical backbone.
Allyl chloride (-CH₂-CHCl-CH=CH-)The C-Cl bond energy is 30% lower than that of a typical chlorine bond, acting like a "weak weld point" on the molecular chain; similar to a "loose screw" on a chain, when the temperature exceeds 140℃ (such as during processing steps like extrusion or injection molding), the chlorine atom at this site will jump out like a flea that has been scalded, leaving behind an "unsaturated double bond."
- Chloro substituent:Chlorine atoms on branched points are more prone to detachment, forming active sites. Similar to "misaligned joints" on a chain, they can be easily "pried open" by oxygen or ultraviolet light, releasing HCl even at relatively low temperatures.
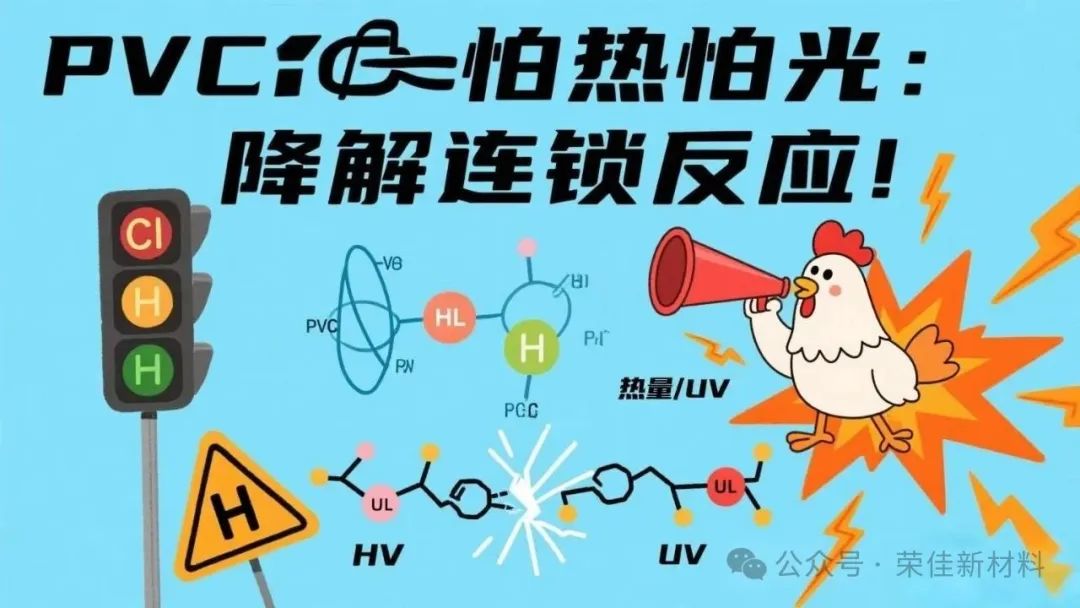
The most troublesome issue is the "vicious cycle": once the first HCl is released, it acts like a "catalyst," encouraging more chlorine atoms to detach along the molecular chain (each release of one HCl can accelerate the detachment of about 10 surrounding chlorine atoms). The originally orderly molecular chain quickly turns into a disordered "conjugated double bond" (its structure is similar to carotene, which is why the material first turns yellow), eventually leading to complete breakage, causing PVC to change from hard and brittle to powder.
In short:
When the temperature exceeds the critical point of 140°C, once a chlorine atom departs, the PVC molecule begins an irreversible self-destruction process.
The released HCl gas becomes a catalytic tool, triggering a chain dechlorination reaction.
The carbon chain at the dechlorination site forms a conjugated double bond structure (-CH=CH-CH=CH-).
The double bonds continue to extend and cross-link, causing the material to change from yellowing to browning and eventually carbonizing and disintegrating.
The sole mission of the stabilizerIt means intercepting the first chlorine atom before it "escapes," thereby blocking this molecular-level "self-destruct program."
2. The "Micro Mechanism of Action" of Three Major Categories of Stabilizers: More Detailed Protective Logic
1. Calcium-zinc metallic soap: like a combination of "fire extinguisher + cleaner"
- Core componentsCalcium stearate (Ca(C₁₇H₃₅COO)₂) and zinc stearate (Zn(C₁₇H₃₅COO)₂).
- Reaction pathway:
- First stage (rapid response): ZnSt₂ reacts with HCl to produce ZnCl₂ and HSt.
- Phase 2 (Risk Control): CaSt₂ reacts with HCl to produce CaCl₂ and HSt.
- Critical rescue (zinc poisoning reversal): CaSt₂ reacts with ZnCl₂ to form the harmless complex CaZn₂Cl₆ and HSt.
- "Blitzkrieg" of zinc soap (zinc stearate):
In the molecular structure, Zn²⁺ carries a positive charge and can quickly capture negatively charged HCl (like a magnet attracting a nail), with a reaction speed more than 10 times faster than calcium soap. In the early stages of processing (when the temperature is at its highest), it can "extinguish the flames" immediately. However, the problem is that after Zn²⁺ and Cl⁻ combine, they form ZnCl₂—this substance can actually accelerate the decomposition of PVC (like pouring oil on a fire during firefighting), so calcium soap must be used to "clean up the aftermath."
- The "protracted war" of calcium soap (calcium stearate):
Calcium soap reacts slowly but has strong endurance; it combines with ZnCl₂ to form a "calcium-zinc composite salt" (similar to putting a "combustion promoter" in a sealed container), continuously neutralizing the subsequently released HCl. The calcium-zinc ratio must be strictly controlled: if zinc:calcium > 1:3, ZnCl₂ will not be processed in time and will form black spots ("zinc burn") on the material's surface; if there is too much calcium, the initial HCl will not be neutralized in time, leading to a chain reaction.
The mechanism of action of PVC heat stabilizers
Organic Tin: A Dual Role as "Surgeon + Shield"
Taking the most commonly used "thiol butyltin" as an example, its molecular structure contains the "Sn-S-R" group.
Active ingredientDimethyltin thioglycolate (R₂Sn(SCH₂COOR')₂).
-Mechanism of action:
- Directly replace the unstable chlorine atom.
PVC-Cl + R₂Sn(SR')₂ → PVC-SR' + R₂Sn(Cl)SR'
- Capture free HCl:
R₂Sn(SR')₂ + HCl → R₂Sn(Cl)SR' + HSR'
- Bug FixesThe Sn atom will actively locate the allylic chlorine on the PVC chain and "pull off" the unstable Cl atom, with its own S atom taking its place (similar to replacing a loose old screw with a new one), thereby stabilizing the molecular chain again.
- Defense functionExcess Sn atoms will act like shields, "wrapping" the free HCl (forming Sn-Cl bonds) and preventing it from damaging other molecular chains.
However, it has two "natural enemies": the phosphorus atoms (P) in phosphorus-containing plasticizers form stable "Sn-P bonds" with Sn (like being handcuffed), preventing Sn from repairing PVC; the fatty acids (high acid value) in recycled materials react with Sn first, effectively "consuming the ammunition in advance."
3. Rare earth stabilizer: like a combination of "reinforcement adhesive + reflective film"
The atomic structure of rare earth elements (lanthanum and cerium) is very special. Their outer shells have many "empty orbitals" that can act like hooks to grasp the allyl chloride groups on PVC chains.
- Reinforcement effectRare earth ions (La³⁺, Ce³⁺) and the Cl atom of allyl chloride form a "coordination bond" (similar to wrapping a loose interface with tape), making it difficult for the chlorine atom to detach.
- Harmless decompositionEven if the rare earth stabilizer is consumed, the decomposition products are stable oxides (such as La₂O₃), which do not catalyze reactions and will not be counterproductive like ZnCl₂.
- Anti-UVThe electronic structure of rare earth elements can absorb ultraviolet light (200~400nm), reducing the "irradiation damage" of sunlight to PVC molecular chains (for ordinary PVC, after one year of exposure to sunlight, the molecular weight decreases by 30%, but with the addition of rare earths, it can be controlled within 10%).

3. The Microscopic Causes of Stabilizer "Failure": Where Did Things Go Wrong?
1. Details of the "coordination error" between calcium and zinc:
- Excess calcium soapCalcium stearate has a large molecular weight and diffuses slowly in the PVC melt. In the early stages, when the concentration of HCl accumulates to a certain level (exceeding 0.01 mol/L), it triggers a chain reaction, causing the material to turn yellow during processing.
-Excess zinc soapThe Zn²⁺ reaction is too fast and can be consumed within 10 minutes. The remaining ZnCl₂ acts like a "catalyst," causing the PVC molecular chains to start breaking at 120°C (normally requires above 180°C), resulting in black spots.
2. The specific process by which organotin is "phased out":
- Phosphorus-containing plasticizers (such as phosphate esters)The P=O bond can form a "coordinate bond" with the Sn atom of organotin (Sn←O=P). The strength of this bond is three times that of the bond between Sn and the Cl atom in PVC, effectively "kidnapping" the tin atom so that it can no longer repair the PVC chain.
- The recycled material with excessive acid value.The thiol groups are prematurely consumed, reducing the protection period by 50%.
3. The microscopic conflict caused by the "disruption" of additives:
- Too much liquid epoxidized soybean oilDue to the polarity difference with calcium-zinc soap, the stabilizer is expelled to the phase interface. It acts like a "lubricant," separating the calcium-zinc soap molecules and preventing them from aggregating on the PVC molecular chain surface (originally, the calcium-zinc soap needed to form a 0.1 μm thick protective layer), causing HCl to directly contact the PVC.
- Lead stearate reacts with sulfurThe sulfur atoms (S²⁻) in sulfur pigments will "capture" lead atoms (Pb²⁺), forming black PbS particles (approximately 50nm in diameter). These particles aggregate on the surface of the material, resulting in black spots visible to the naked eye.
- Certain additives (such as β-diketones)Decomposes when heated, producing crystals that cause the surface of the product to appear "frosted" (like covered with a layer of white frost).
Stabilizer Formula Failure – Concise Version
▼The "relay discontinuity" of metallic soaps
- Excessive use of calcium soap → delayed reaction → initial accumulation of HCl triggers a chain reaction;
- Excessive proportion of zinc soap → overconsumption → unchelated ZnCl₂ catalyzes "zinc burn" black spots.
▼ The Active Seal of Organotin
- The formulation incorporates a phosphorus-containing plasticizer → Phosphorus atoms form P-Sn bonds with tin → Loses the ability to replace chlorine.
Recycled material with excessive acid value → Premature consumption of thiol groups → Protection period shortened by 50%.
▼ Thermodynamic traps of migration precipitation
- Excess liquid epoxidized soybean oil → polarity difference with calcium-zinc soap → stabilizer is expelled from the interface.
Lead stearate reacts with sulfur-containing pigments to form black PbS precipitate, resulting in sudden black spots on the product.
- Thermal decomposition of β-diketones → Release of malonic acid derivatives → Surface crystallization efflorescence.
Through this more detailed mechanistic analysis, it becomes clearer that stabilizers are not "cure-alls," but rather they precisely intervene in the decomposition pathways of PVC molecules to remedy structural defects—much like patching up cracks in a leaky dam one by one. Any misstep in coordination could lead to the collapse of the protective system.The evolution of stabilizers is a dance between materials science and chemistry; the most efficient stabilization enables molecular chains to self-repair, and the smartest formulation ensures every atom precisely finds its place.A 0.1% difference in composition may determine the service life of PVC products.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






