A guide to understanding defoamers
1. The Formation of Foam
When surfactants are present in a liquid, the molecules can adsorb onto the bubble surface. Once these molecules, oriented on the bubble surface, reach a certain concentration, they form a robust bubble wall film. The surfactant molecules adsorb at the gas-liquid interface to form a liquid film, reducing the surface tension and thereby increasing the gas-liquid contact area, which makes it difficult for bubbles to coalesce. Air bubbles have a much lower density than water, so they rise to the surface of the liquid. When the rising bubbles pass through the liquid surface, they adsorb the surfactant molecules from the upper layer of the surface. Therefore, the bubble film exposed to the air, with adsorbed surfactant, differs from the bubble film in the solution; it is covered with two layers of surfactant molecules. The adsorbed surfactant provides a protective function for the liquid film, and the hydrophobic groups of the second layer of surfactant molecules face the air, as shown in Figure 1.
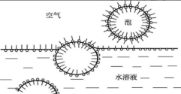
2. The Harm of Bubbles
The production capacity is greatly limited: for example, in various biological fermentation processes (such as beer production, alcohol manufacturing, and the production of major antibiotics in medicine), various fermentation tanks, reactors, and cooking vessels. To prevent the occurrence of foam and avoid overflow losses, the feed ratio has to be significantly reduced.
2. Waste of raw materials and products: Due to foam, valuable or useful raw materials may overflow and be lost, resulting in obvious waste.
3. Extended reaction cycle: The presence of gases and liquids among the chemical reaction products causes gas retention due to foam, prolonging the reaction cycle and unnecessarily consuming more energy. Additionally, if foam causes over-fermentation in wine, it may alter the flavor...
4. Impact on product quality: In the textile industry, during the processes of dyeing, printing, and aqueous coating, the retention of bubbles leads to blemishes and defects on finished fabrics. In the case of pulp slurry, foam not only poses a hazard to environmental hygiene and worker health but also results in numerous holes in the finished paper, thereby causing a significant decline in product quality.
5. Not conducive to accurate measurement: In industrial processes, the presence of foam interferes with the accurate measurement of liquid level gauges, leading to measurement errors. The presence of foam in the liquid causes significant fluctuations in liquid density, which can often result in falsely high liquid levels in reactors, absorption towers, and distillation columns, causing operational imbalance and even leading to accidents.
One of the causes of environmental pollution and accidents: Due to foam overflow, it will inevitably pollute the production environment and its surrounding environment, and in some cases, even cause major accidents.
The above is by no means the entirety of the harm caused by bubbles, but it is enough to demonstrate their severity. In short, the existence of bubbles affects various sectors and aspects of the national economy. If not properly addressed, it is no exaggeration to say that "bubbles" will become a roadblock for us and a "bottleneck" in certain processes. It is reassuring that we already have good strategies for eliminating bubbles.
3. Defoaming Mechanism
To eliminate foam, methods such as settling, heating, or decompression are generally used, but these are time-consuming. In various production processes and product applications, it is necessary to quickly eliminate the generated foam, which requires the use of defoamers. Therefore, it is necessary to understand the mechanism of action of defoamers.
Theoretically, eliminating the factors that stabilize foam can achieve defoaming. The primary factor affecting foam stability is the strength of the liquid film. When a defoamer is added to the working solution, it becomes a solution, emulsion, or dispersion that adsorbs onto the foam surface. Since it has a lower surface tension than the foam, it can attract surfactants or foaming substances, reducing the surface viscosity of the foam liquid film, causing local thinning and rupture. Therefore, the main reasons for foam destruction are the thinning of the liquid film and the diffusion of gas within the foam. To prevent the reformation of foam, surface elasticity should be reduced or eliminated, that is, utilizing a brittle surface film instead of an elastic one, resulting in unstable foam.
4. Types and Characteristics of Defoamers
For defoamers, we hope to achieve both defoaming and foam suppression effects, with low solubility in water, and they should not be easily emulsified or solubilized by the working fluid. There are many types of defoamers, but generally, they can be divided into two categories: silicone-containing and non-silicone.
01
Silicone defoamer
Silicone-based defoamers have low surface tension, low solubility, good dispersion, long-lasting effect, and require a small amount, which gives them strong foam-breaking and foam-suppressing capabilities. Additionally, these defoamers are chemically inert, non-toxic, and environmentally friendly. They mainly include silicone oil, silicone oil emulsions, silicone oil solutions (silicone oil dissolved in organic solvents), and silicone oil with other fillers (such as SiO2, Al2O3), among others.
02
Silicone-free defoamer
There are the following types based on different components:
(1) Phosphate ester defoamers: They have good defoaming effects and are widely used.
(2) Alcohols, ethers, fatty acids and their esters, animal and vegetable oils or mineral oils, as well as substances like polyethylene glycol and propylene glycol: The characteristics include easy availability of raw materials and a certain defoaming effect. A small amount is used alone, but most are used in combination.
(3) Polyether-based defoamers: Adducts of alcohols and ethylene/propylene oxide. By controlling the carbon content of the alcohol and the ratio of ethylene/propylene oxide, its defoaming performance can be controlled. This type of defoamer is characterized by the ability to develop some high-temperature resistant products. Since it does not contain silicone, it will not cause contamination or oil stains on equipment walls and fabrics. Some products not only have defoaming and foam suppressing effects but also serve as functional additives in the textile industry, playing roles in penetration, washing, retard dyeing, and leveling, making them more widely applicable than silicone-containing defoamers.
Points to consider when choosing a defoamer:
1. Insoluble or slightly soluble in foaming liquid.
Lower surface tension than the foaming liquid.
3. Has a certain degree of affinity with the foaming liquid.
4. Does not undergo a chemical reaction with the foaming liquid.
Low volatility and long-lasting effect.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






