99.5% selectivity! academician jiang jianchun's team: Solvent-Regulated Precise Catalytic Conversion of HMF
5-Hydroxymethylfurfural (HMF), a biomass platform molecule, is recognized by the U.S. Department of Energy as one of the "Top 10+4" key platform compounds due to its structure containing functional groups such as aldehyde, hydroxymethyl, and furan ring. It shows great potential for conversion into high-value products such as BHMF, DMF, BHMTHF, and 1,6-HDO.
However, the hydrogenation process of HMF has a complex pathway involving multiple intermediates and products. Achieving precise control over the reaction direction in a single catalytic system remains a challenging task.
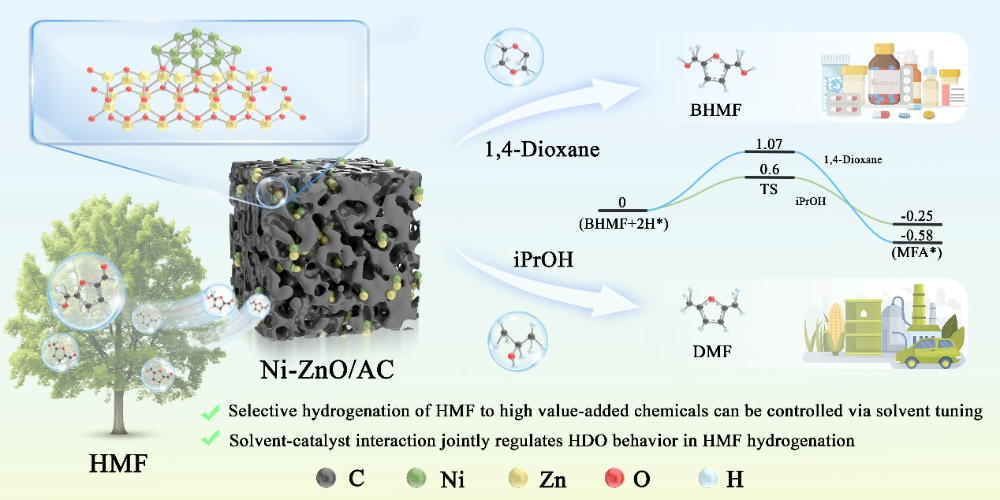
In response to the challenge of the existing catalytic systems' difficulty in precisely controlling product selectivity, the team led by Academician Jianchun Jiang from the Institute of Chemical Industry of Forest Products, Chinese Academy of Forestry, has innovatively developed a Ni-ZnO/AC coupled solvent multiphase catalytic system using a low-temperature co-precipitation method. This system achieves high selectivity conversion of HMF under a single catalyst system—preferentially producing BHMF in 1,4-dioxane with a selectivity of up to 97.5%, and directionally converting to DMF in isopropanol with a selectivity of up to 99.5%.

The related research results are presented in "A Promising Strategy for Solvent-Regulated Selective Hydrogenation of 5-Hydroxymethylfurfural over Porous Carbon-Supported Ni-ZnO Nanoparticles." In "Nano-Micro Letters" (IF 36.3) 。
This study systematically reveals for the first time the decisive regulatory effect of solvent polarity and proton donor ability on the product pathway of HMF hydrogenation. The highlights of this paper are as follows:
A composite catalyst of Ni-ZnO nanoparticles supported on coconut shell carbon (Ni-ZnO/AC) was successfully constructed using a low-temperature co-precipitation method, demonstrating excellent catalytic activity and cycle stability in HMF hydrogenation reactions.
By regulating the solvent system, high selective conversion of HMF under a single catalyst system is achieved—preferentially producing BHMF in 1,4-dioxane (with a selectivity of up to 97.5%) and directionally converting to DMF in isopropanol (with a selectivity of up to 99.5%).
The system reveals the synergistic regulation mechanism of reaction pathways by solvent polarity and proton donor ability, proposing a new hydrogenation-deoxygenation strategy based on the hydrogen shuttle effect, providing theoretical support for the precise and high-value utilization of biomass platform compounds.
In this work, dried coconut shells with a particle size of 4–5.6 mm were selected as raw materials, and a porous carbon-loaded Ni-ZnO nanoparticle catalyst (Ni-ZnO/AC) was prepared using a low-temperature co-precipitation method.
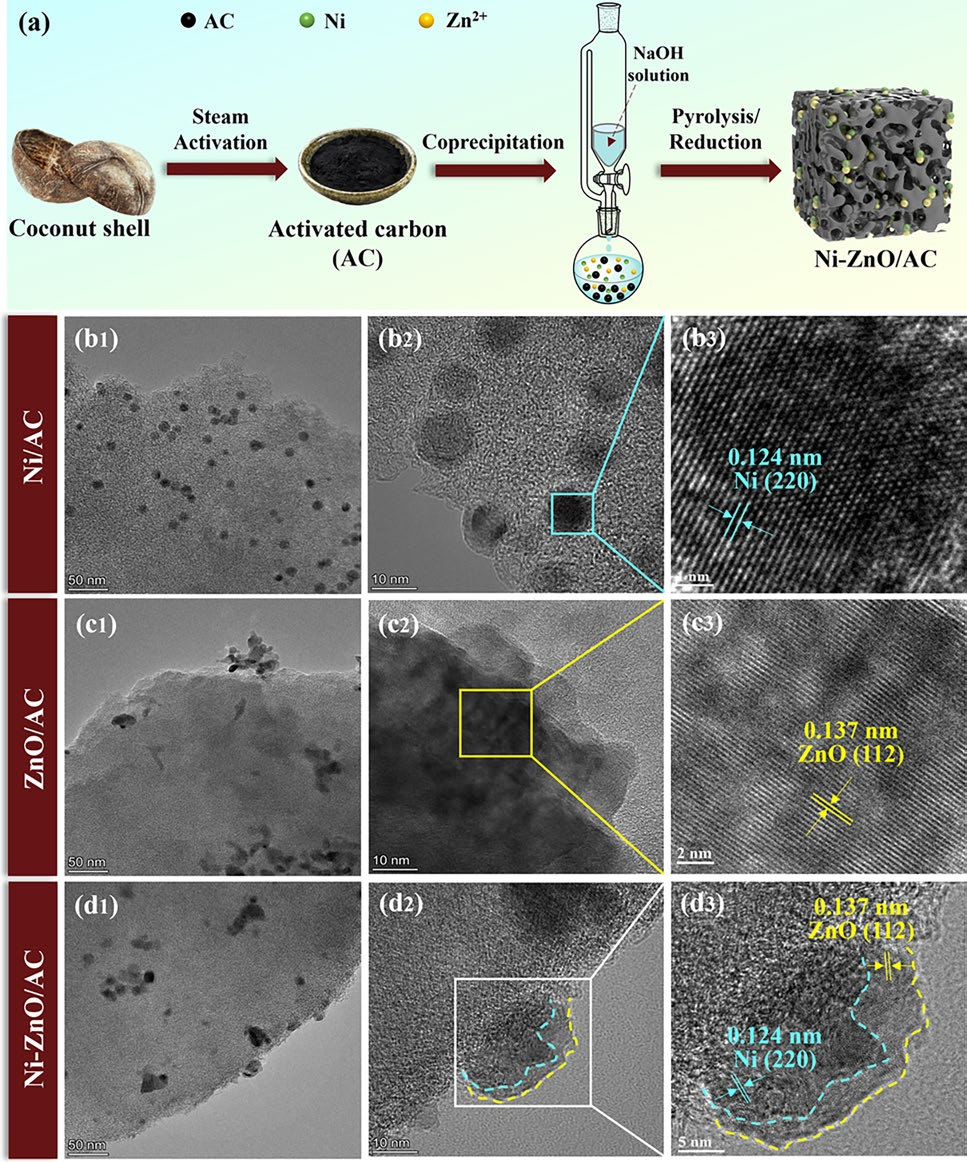
Figure 1 The synthesis process and microstructure characterization of Ni-ZnO/AC. a Synthesis steps of Ni-ZnO/AC. b TEM image of Ni/AC, c TEM image of ZnO/AC, and d TEM image of Ni-ZnO/AC.
02
Compared to 9 types of solvents, the conversion rate of isopropanol reaches 99.5%.
HMF, due to its multi-functional group structure, can undergo hydrogenation reactions in solvents to be converted into various high-value-added compounds.
This study systematically examined the effect of Ni-ZnO/AC catalyst on four types of polar aprotic solvents and five types.Polar protic solventThe effect of hydrogenation reaction on HMF.
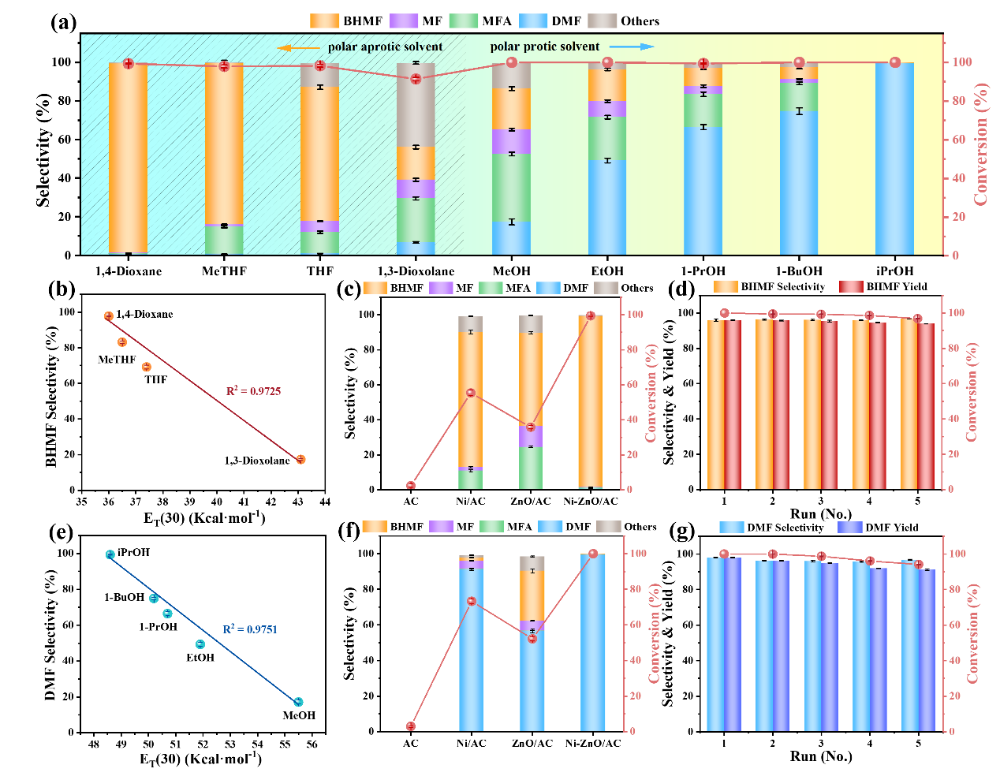
Figure 2The effect of solvent types and polarity on the catalytic hydrogenation of HMF and the recycling performance of Ni-ZnO/AC catalyst
Four types of polar aprotic solvents: 1,4-dioxane, 2-methyltetrahydrofuran, tetrahydrofuran, 1,3-dioxolane.
5 types of polar protic solvents: methanol, ethanol, 1-propanol, 1-butanol, isopropanol
The results show that in polar aprotic solvents, HMF preferentially forms BHMF.2,5-FurandimethanolThe selectivity is as high as 97.5% especially in 1,4-dioxane.
In polar aprotic solvents, it tends to form DMF (2,5-DimethylfuranThe selectivity in isopropanol is as high as 99.5%.
Further analysis revealed that the selectivity of BHMF and DMF is linearly positively correlated with the solvent polarity ET(30) value, indicating that solvent polarity can significantly enhance the solubility of the substrate and the degree of reaction completion, thereby affecting the reaction pathway and product distribution.
In addition, the catalyst maintains good stability in the recycling process within two solvent systems.
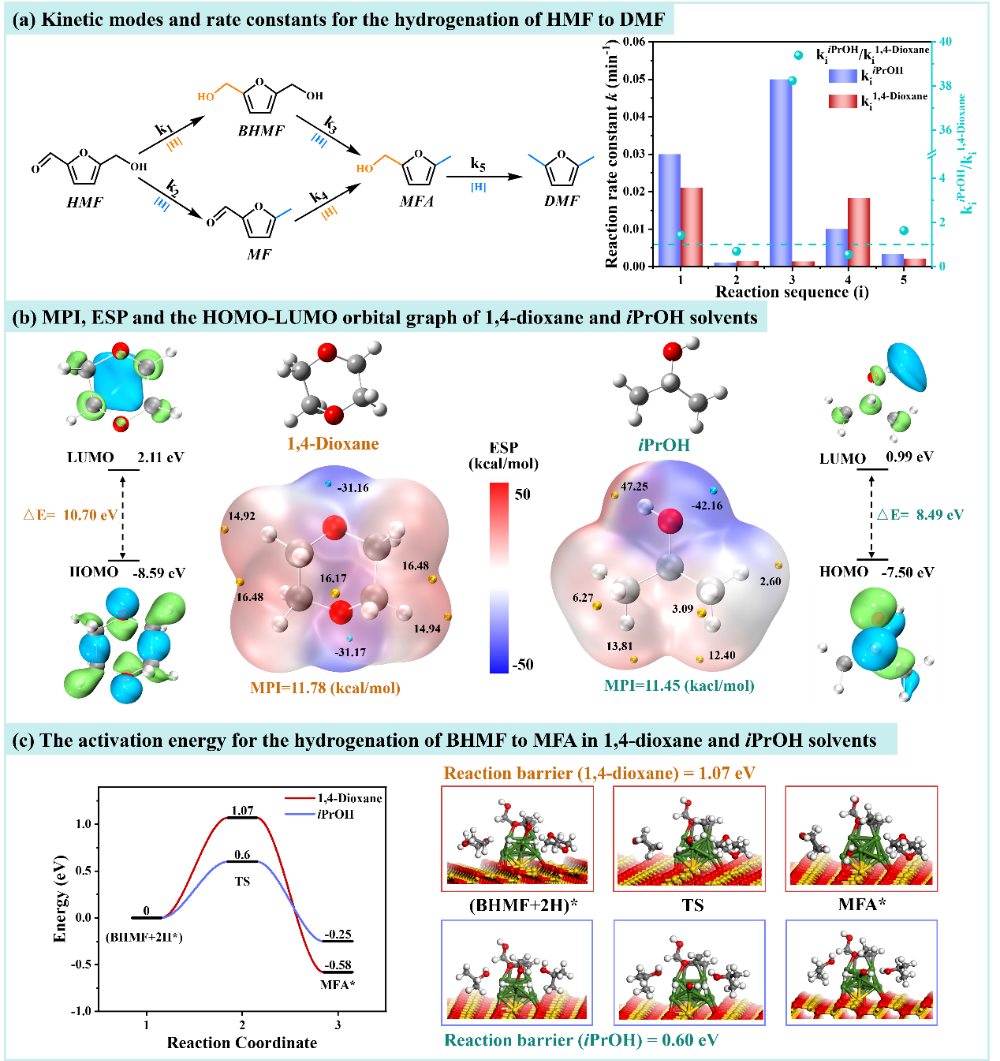
Figure 3 Kinetics, molecular properties, and energy barrier analysis of HMF hydrogenation in 1,4-dioxane/isopropanol solvent.
In isopropanol, the rate constant (k) for the conversion of HMF to BHMF1The rate is 1.5 times higher in 1,4-dioxane, indicating that protic solvents are more favorable for carbonyl hydrogenation under the action of the Ni-ZnO/AC catalyst.
The step of further converting BHMF to MFA (k3In isopropanol, it is 38.2 times that of the latter, indicating that this process is almost inhibited in 1,4-dioxane, BHMF tends to accumulate, and the product selectivity naturally favors BHMF.
Subsequently, by comparing the molecular polarity index (MPI), electrostatic potential distribution (ESP), and HOMO-LUMO energy gap (ΔE) of the solvents, it was found that although 1,4-dioxane has a slightly higher polarity, it lacks proton donor capability, making it difficult to facilitate the crucial proton transfer.
Isopropanol not only has a smaller ΔE and stronger charge polarization capability, but it can also directly provide protons and stabilize reaction intermediates, offering strong support for subsequent dehydroxylation and hydrogenation processes.
Furthermore, DFT calculations based on the explicit solvent model reveal that the hydrogenolysis reaction of BHMF on the Ni-ZnO/AC surface has a barrier of only 0.60 eV in an isopropanol system, which is significantly lower than the 1.07 eV in a 1,4-dioxane system.
Isopropanol participates in the reaction through the "hydrogen shuttle effect," not only does its proton accelerate the departure of the hydroxyl group, iPrO...-It can also capture active hydrogen on the Ni surface, achieve solvent self-regeneration, and complete an integrated cycle.
Although solvent polarity has some influence on the regulation of reaction products, it is the proton donor ability that is the key factor determining the reaction pathway and product selectivity.
Polar protic solvents represented by isopropanol not only enhance the solubility of substrates and the contact with catalysts but also actively participate in the reaction through hydrogen bonding and proton transfer processes, greatly promoting the highly selective conversion of HMF to DMF.
Summary and Outlook
This study constructed a Ni-ZnO/AC heterogeneous catalyst coupled with different solvent catalytic conversion systems, achieving precise conversion of the biomass platform molecule HMF to high value-added products.
In 1,4-dioxane/isopropanol, BHMF and DMF are generated with high selectivity, respectively.
The study, combining experimental results and DFT calculations, found that the solvent not only serves as a reaction medium but also forms a synergistic effect with the catalyst, profoundly influencing the reaction rate and path selection.
This strategy effectively manipulates product distribution by regulating solvent polarity and hydrogen-donating ability, providing a controllable new approach for green chemical transformations.
This research work was supported by the National Natural Science Foundation of China's Outstanding Youth Fund (32222058) and the Fundamental Research Funds of CAF (CAFYBB2022QB001). Huang Rulu, a doctoral student from the 2022 class at the Institute of Chemical Industry of Forest Products, is the first author. Academician Jiang Jianchun and Researcher Wang Kui from the Institute of Chemical Industry of Forest Products, Researcher Tian Ziqi from the Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, and Professor Chai Yongming from China University of Petroleum (East China) are the co-corresponding authors.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






