Zhejiang University Li Zhenglong Team: Photocatalytic Oxidative Depolymerization of Polyethylene Plastic Waste into Aliphatic Long-Chain Dicarboxylic Acids
Recently, the global annual production of polyethylene (PE) has reached 83 million tons, making it one of the most widely used plastics. The long-term accumulation of polyethylene waste poses a serious threat to the environment. This study presents a method for catalytically oxidizing polyethylene (PE) using BiOI/BiVO4 p-n heterojunction materials under visible light irradiation to generate valuable aliphatic dicarboxylic acids (C4-C30). The method achieves a carbon yield of up to 83% under mild conditions, and by optimizing the composition of the heterojunction and reaction conditions, the yield of long-chain dicarboxylic acids (C10-C30) can be enhanced to 75%. The authors also investigate the effects of reaction temperature, time, air pressure, and the stability of the catalyst after multiple cycles on the photocatalytic oxidation of PE. Furthermore, the reaction mechanism is elucidated using various technical approaches: superoxide radicals (•O2-) are responsible for activating the C-H bonds in the PE backbone, while hydroxyl radicals (•OH) cleave the C=C bonds. Additionally, the photocatalytic upgrading process shows significant efficiency for various commercial PE types used in daily life. This photocatalytic system offers a promising method for the efficient recycling of PE waste.
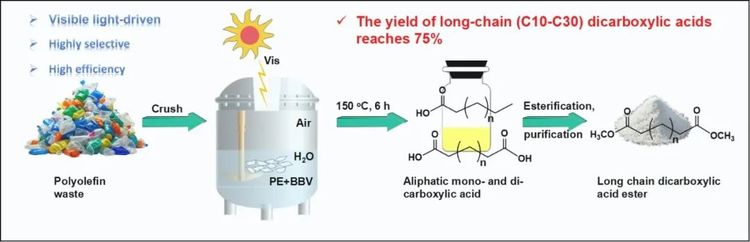
Figure 1. Schematic illustration of the photocatalytic oxidation depolymerization of polyethylene waste plastics into dicarboxylic acids proposed in this study.
Background introduction:
As the amount of plastic waste continues to increase, the efficient recycling of plastics has become a severe environmental issue. Especially for plastics like polyethylene (PE), due to their stability and durability, the degradation rate in the environment is very slow, posing a long-term threat to ecosystems. Currently, there are some limitations to the chemical recycling methods for plastics, particularly polyethylene, such as high energy consumption processes and the issue of secondary pollution generated. To address these problems, scientists are seeking more environmentally friendly and energy-efficient methods for plastic recycling. Photocatalytic systems have emerged as an attractive option due to their use of renewable solar energy and relatively low operational conditions. In recent years, progress has been made in the degradation and recycling of plastic waste using photocatalytic systems, with researchers aiming to simulate the natural degradation mechanisms through reactive species such as free radicals, thereby achieving green recycling of polyolefin waste. BiOI/BiVO4 p-n heterojunction materials have been proposed as photocatalysts due to their stability and non-toxicity. In this study, researchers developed this material and successfully achieved photocatalytic oxidation of polyethylene under mild conditions using visible light irradiation. This research contributes to the search for environmentally friendly and low-energy plastic recycling technologies, utilizing the photocatalytic performance of BiOI/BiVO4 p-n heterojunction materials to efficiently convert polyethylene waste into higher value chemicals.
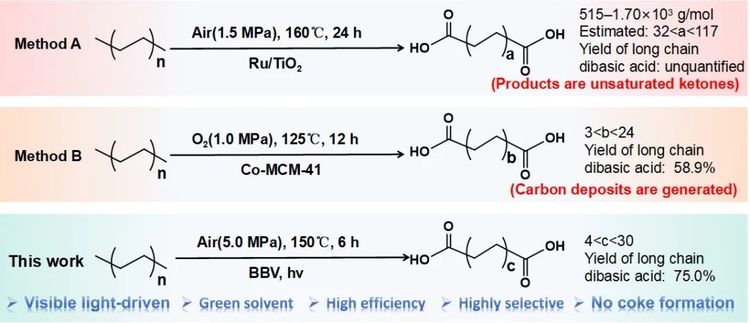
Figure 2. Comparison of this catalytic system with existing methods.
Highlights of this article:
This work successfully achieved the photo-catalytic oxidation of polyethylene (PE) to valuable aliphatic dicarboxylic acids (C4-C30) under mild conditions using BiOI/BiVO4 p-n heterojunction photocatalyst, with a carbon yield up to 83%. Notably, the carbon yield for long-chain dicarboxylic acids (C10-C30) reached 75%.
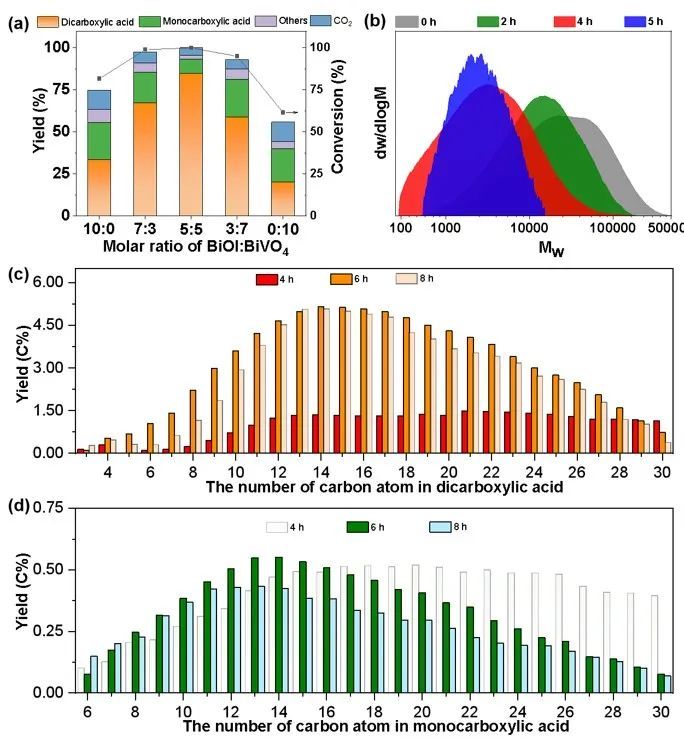
Figure 3. (a) Reaction results of PE on different catalysts, (b) molecular weight of PE, (c) dicarboxylic acids, (d) monocarboxylic acid product distribution over time.
In the photocatalytic oxidation process, superoxide radicals (•O₂⁻) and hydroxyl radicals (•OH) play a crucial role. The superoxide radicals activate the C-H bonds in the PE main chain, while the hydroxyl radicals oxidize and cleave the C=C bonds, ultimately converting PE into dicarboxylic acid products.
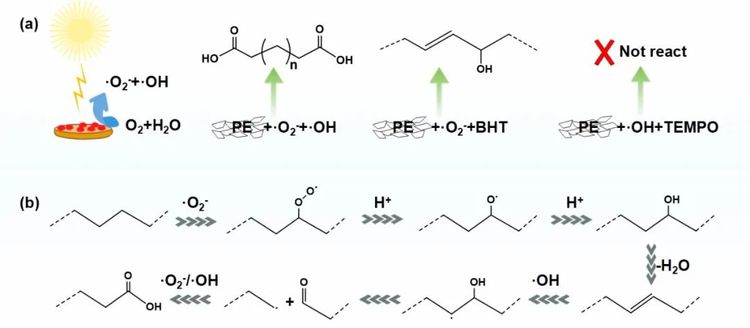
Figure 4. Proposed reaction mechanism
This catalytic system can oxidatively depolymerize most commercial PE into dicarboxylic acids, with carbon yields of the dicarboxylic acids ranging from 59% to 64%. Small amounts of branched alkanes and cycloalkanes were also detected in the products. The photocatalytic system exhibits excellent stability when processing commercial PE waste, indicating its potential for practical applications.
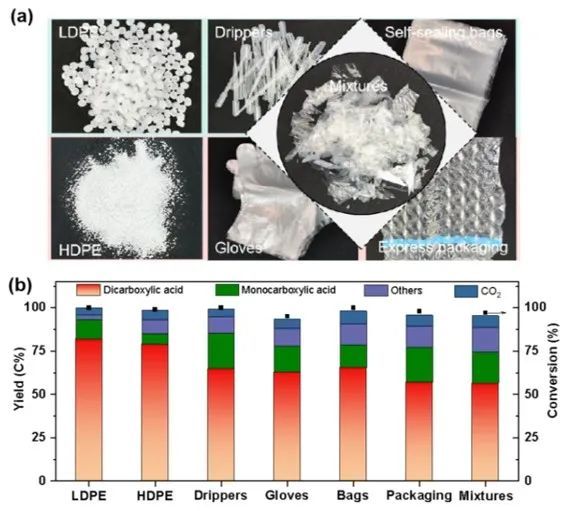
Figure 5. Photocatalytic oxidation depolymerization results of pure PE particles and common PE waste plastics in daily life
Summary and Prospects:
Developing green chemical methods for the efficient recycling of waste polyolefins is essential for reducing carbon emissions and supporting sustainable development. By converting polyethylene (PE) into valuable chemicals (long-chain dicarboxylic acids), the recycling of waste plastics can be enhanced, providing raw materials for the production of biodegradable polymers, lubricants, and surfactants. The photocatalytic oxidation method proposed in this study operates under mild conditions, significantly reducing the energy and resource inputs required by traditional methods, and is expected to advance the development of recycling technologies for polyolefin waste plastics.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






