Efficiently Recycle PET Using Air Moisture, with an Efficiency Rate of Up to 94%
Polyethylene terephthalate (PET) is one of the most widely used plastics, crucial in beverage bottles, food packaging, and textiles. With an annual global production approaching 70 million tons, PET accounts for 67% of the market share in the beverage industry. However, excessive use and low recycling rates mean that PET makes up 12% of solid waste.
A research team from Northwestern University in the United States has developed a groundbreaking method to break down PET without using toxic chemicals, excessive heat, or harsh solvents. Their approach utilizes trace amounts of moisture in the air to selectively decompose PET into its original monomers, which can then be recycled into new plastics or even upgraded into higher-value materials.
"America is the country with the most severe per capita plastic pollution, and we only recycle 5% of our plastics," said Yosi Kratish, the lead researcher on the project. "There is an urgent need for better technologies to handle different types of plastic waste. Most methods today involve melting plastic bottles and then downcycling them into lower quality products. What is particularly exciting about our research is that we use moisture from the air to break down plastics, achieving an exceptionally clean and selective process."
The new process uses a simple, abundant molybdenum catalyst with activated carbon as the carrier. The PET plastic is exposed to this catalyst and heated to 250-265°C, which is just slightly above the melting point of PET. Once the chemical bonds of the plastic are weakened, exposure to air completes the decomposition process, converting PET into terephthalic acid (TPA), a valuable chemical used for making new plastics. Unlike traditional methods, this process produces almost no waste byproducts—only acetaldehyde, an industrial chemical that is easy to remove.
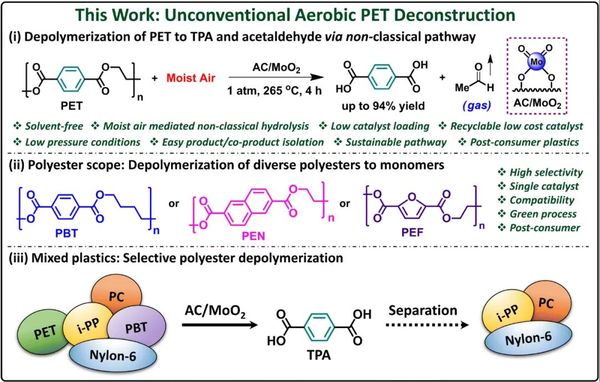
This method has several advantages over existing recycling technologies. First, it eliminates the need for expensive and toxic solvents, reducing environmental impact. The reaction is also highly selective, meaning it only breaks down PET without affecting other plastics. This eliminates the costly and time-consuming need to sort plastic waste before recycling.
"There are many drawbacks to using solvents," Klatish explained. "They can be expensive, and you have to heat them to high temperatures. Then, after the reaction, you end up with a bunch of materials that must be sorted to recover the monomers. Instead of using solvents, we used water vapor from the air. This is a more elegant way to solve the problem of plastic recycling."
The speed of the process is another major advantage. Traditional chemical recycling methods may take up to 72 hours, while Northwestern University's method can nearly completely decompose PET in just four hours. The catalyst itself is also durable and reusable, maintaining its effectiveness over multiple recycling cycles.
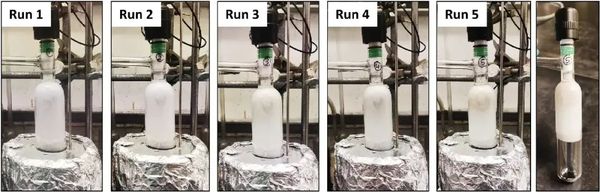
The photo shows the formation of TPA products after each 4-hour heating in different runs (total reaction time = 20 hours).
"The results show that the process is fast and effective," said Naveen Malik, the first author of the study. "After just four hours, 94% of TPA was recovered. The catalyst is durable and recyclable, meaning it can be used over and over again without losing its efficacy."
In addition, the method is applicable to real-world plastic waste. Researchers tested it on plastic bottles, polyester fibers, and even mixed plastic waste, finding that it successfully extracted pure, colorless TPA from colored plastics. Unlike other methods for PET that require pre-cleaning, this process works even on contaminated plastic waste.
The Northwestern University team is now working to scale up the process to handle industrial-scale plastic recycling. With further optimization, they believe this technology can significantly reduce global plastic pollution and contribute to the development of a circular economy, allowing plastics to be continuously reused rather than discarded.
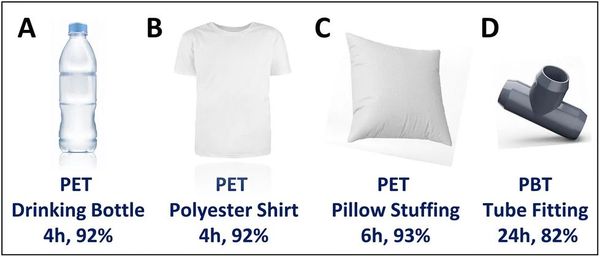
Aerobic solvent-free AC/MoO2-mediated degradation of post-consumer plastic materials A-D. Reaction conditions: 100 mL Schlenk flask, post-consumer polyester stored in air, AC/MoO2 (3.24 wt% Mo), solvent-free, 265 °C, air. Yield determined by 1H NMR using m-xylene as an internal standard.
"Our technology has the potential to significantly reduce plastic pollution, lower the environmental footprint of plastics, and contribute to the development of a circular economy, allowing materials to be reused rather than discarded," Malik said. "This is a tangible step towards a cleaner, greener future, demonstrating how innovative chemistry can address global challenges in a way that is in harmony with nature."
If adopted on a large scale, this technology can help address one of the world's most pressing environmental challenges—plastic waste. By eliminating the need for harsh chemicals and excess energy, this method makes recycling more practical, cost-effective, and environmentally friendly.
As millions of tons of plastic waste continue to accumulate each year, such innovations can play a key role in building a more sustainable future.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics






