Why "Interface" Dictates the Lifeline of Composite Materials
Have you ever encountered such a situation:
Even though high-strength carbon fiber was used, the material still cracked.
The performance of the fiberglass-reinforced PP selected is worse than that of pure resin.
The Tg is very high, but it started peeling and cracking structurally in less than two years...
Many people's first reaction is to doubt whether "the materials are not good enough." In fact, the problem often lies not in the materials themselves, but in the "layer" between the materials—the interface.
The one thing that composite materials dread the most is when the reinforcement is very strong, the matrix is also not weak, but the interface is a mess.
So today, let's clarify why the interface is truly the lifeline of composite materials.
1. The interface is not an edge, but a "physical field"
When many people hear the term "interface," what likely comes to mind is the boundary where Material A and Material B meet.
However, in modern materials science, this understanding is far from enough.
Today, the "interface" we are talking about is no longer just the "boundary line" between two materials, but a more complex and crucial area. It is a small "intersection zone," yet it involves the convergence of multiple physical mechanisms and multi-level structures.
It is neither the matrix nor the reinforcing phase, but a "transition zone" between the two.
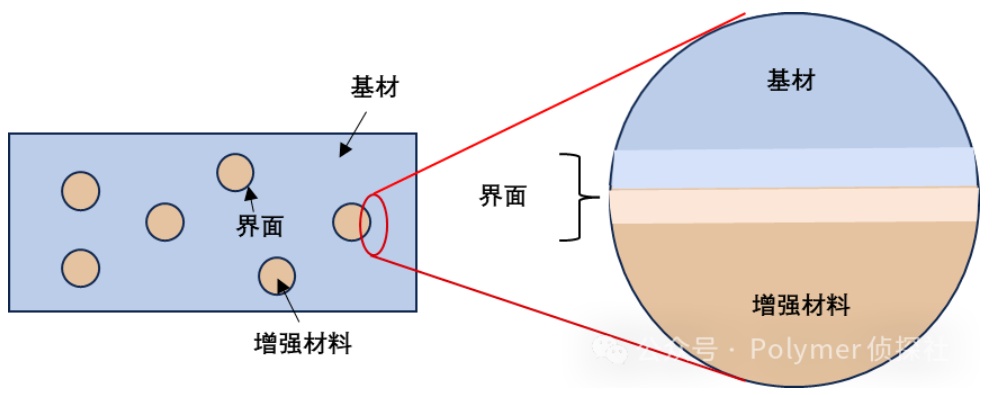
It has a lot of functions, such as:
It is the bridge for stress transfer: whether the load you apply can be effectively transmitted from the matrix to the reinforcement material depends entirely on this.
It is a buffer layer for energy transition: molecular motion, heat conduction, reaction activity, etc., all gradually decline or connect in this area.
It is an interleaved region of microstructures: here, amorphous regions, ordered structures, and reaction products often coexist, forming a "mixed structure."
It is also the root of adhesion: whether the interface sticks firmly or not depends on the intermolecular forces, chemical bonds, physical interlocking and other mechanisms in this area.
In summary, it is:
The interface is not a seam; it's a critical corridor where the compatibility of materials is determined.
"Composite materials are not 'splicing,' but 'synergy.'"
Many people understand composite materials as "building blocks":
Fibers are responsible for strength, the matrix is responsible for shaping, and combining them does the trick.
However, materials are not about such "arithmetic addition". A true composite material must meet a key condition:
All components must "work together" - being able to bear force together, deform together, and carry loads together, instead of moving or sliding independently.
This crucial bridge relies on an interface.
Here's an extreme example for you to understand:
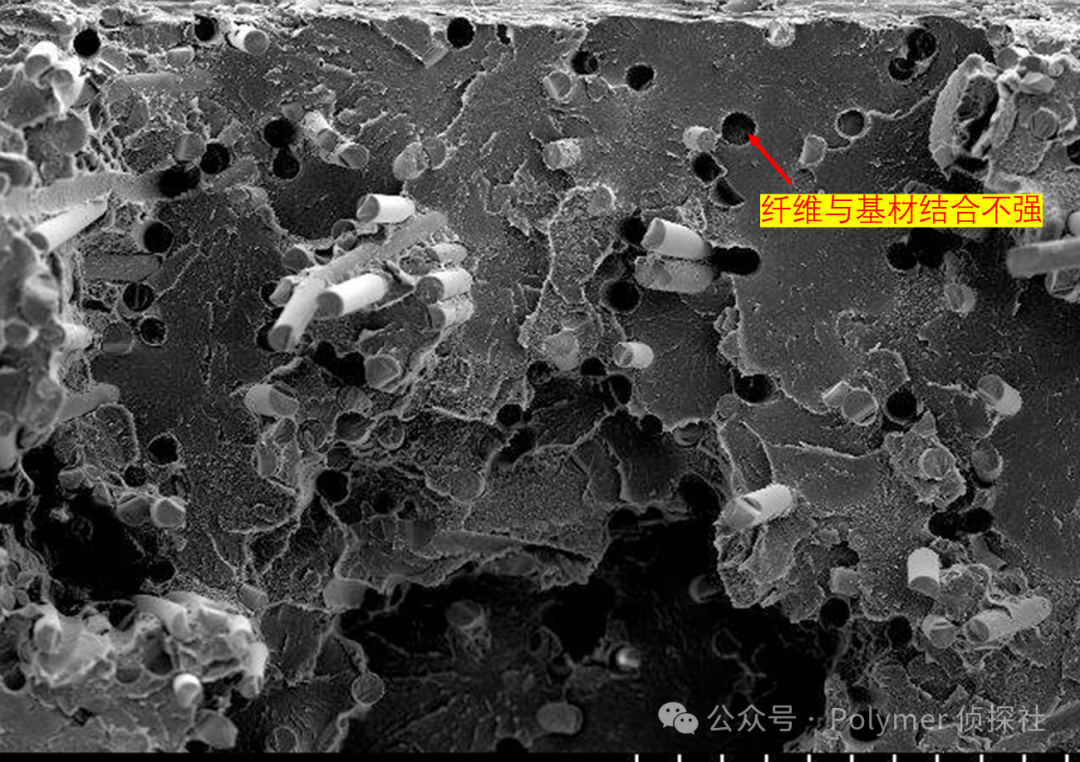
If you use carbon fiber-reinforced resin and the carbon fiber and matrix "separate," the load cannot be transferred to the fiber at all, resulting in the resin cracking first and the fiber spinning idly — complete failure.
Conversely, if the interface is properly designed, the matrix can steadily transfer the load to the fibers, and the fibers can also help prevent the propagation of cracks, thereby enhancing the overall performance of the material.
So we say:
The strength of composite materials does not come from the strength of a single component, but because they "pull together."
A poor interface can lead to what problems?
If the interface is not well designed, it will become the "source of cracks" in the entire structure. This is not an exaggeration, but a matter of experience.
Common interface failure modes:
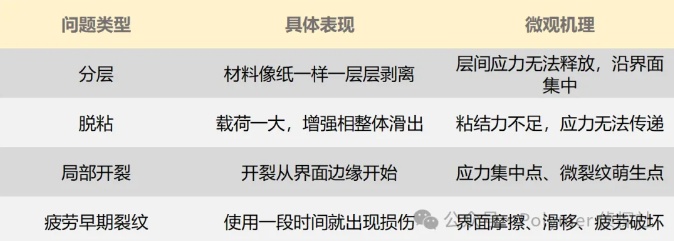
These questions all point to a commonality:
Once the interface "splits," the materials will fall apart.
Modern Interface Science: From Surface Energy to Multi-Field Coupling
After discussing the engineering issues, let's elevate our perspective.
Today's interface research has long surpassed the era of "how well it sticks" and has entered a more detailed and systematic stage of study. Friends working on composite materials can delve deeply into these three models.
1. Interface Interaction Spectrum (IIS)
In the cutting-edge views of materials science, interfacial adhesion is no longer simply a matter of "whether it is bonded or not," but can be decomposed into a complete "spectrum of energy interactions."
This pedigree includes several major molecular-level forces:
Covalent bond: For example, silane coupling agents can bridge a true "chemical bond" between fillers and resins.
Hydrogen bonds/π-π stacking: Such as the mutual attraction between aromatic rings or the hydrogen bonding between hydroxyl groups.
Electrostatic interactions: such as the attraction between charged groups, for example, between ionic fillers and polar resins.
Dispersion force/Van der Waals force: This is the most fundamental but weakest interaction, mainly present between non-polar interfaces, such as PP and some non-polar fillers.
The strength and characteristics of each type of force directly influence whether an interface is "sticky" or "non-sticky." For different material combinations, it is essential to identify the "dominant force" to precisely design the interface and achieve true synergy.
Sure, here is the translation: "Provide an example:"
When reinforcing epoxy resin with carbon fiber, it is best to introduce aromatic structures or hydroxyl groups onto the surface of the carbon fiber, as they can form π-π stacking or hydrogen bonds with the epoxy system, enhancing the "soft intimate contact."
Glass fiber reinforced polypropylene (PP) has one strong polarity and one weak polarity, making them naturally "incompatible." At this point, it is necessary to introduce a coupling agent (such as maleic anhydride grafted PP) to provide a polar "bridge" for effective bonding. (This was a topic from a friend's comment a few days ago.)
2. Stress Transmission Mechanism: From "Shear Lag" to "Decoupled Cooperation"
In traditional composite material mechanics models, such as the famous shear-lag model, we usually assume:
The load is transferred from the matrix to the reinforcing fibers through "shear forces" at the interface. This is a concept of "pulling and tugging, firmly holding on."
However, in recent years, more and more studies have found that this understanding is too idealistic. The interface in reality is not "stuck" there motionless, but rather a controllable slip area.
Five, New Perspective: Sliding is not a bad thing; it is actually a pressure relief valve!
When a material is subjected to force, if the interface allows for a certain degree of micro-slip, it can actually mitigate stress concentration and prevent the material from brittle fracture at the interface.
Some advanced designs even intentionally make the interface into an "energy-dissipating band"—when stress occurs, the interface absorbs and dissipates a portion of the energy, thereby enhancing the overall impact toughness.
It's like putting on shoes:
Tight shoes can chafe the feet and hinder long walks (a hard interface can be brittle and break easily).
The shoes are too loose, easy to fall off and unstable (the interface is too slippery and unable to transmit force);
The ideal state is to be "neither too tight nor too loose"—able to stand firmly while also being flexible enough to move.
This is precisely what modern composite materials aim for: the interface must stabilize stress while providing appropriate buffering and decoupling, avoiding a "rigid confrontation" and instead achieving a "flexible synergy."
3. Smart Interface Design
The interface of composite materials is undergoing a revolution from "passive adaptation" to "active regulation."
The most cutting-edge trends are:
No longer satisfied with letting the interface "silently support," but instead giving it "thinking ability" - actively adjusting its own state based on external stimuli. This is what is known as Smart Interface design.
They can "understand signals" and respond autonomously.
Thermal Response Interface: Upon heating, the material undergoes molecular reorganization, enabling the interface to self-repair, such as the automatic healing of microcracks.
Light-responsive interface: Under ultraviolet irradiation, the molecular structure changes, allowing reversible switching of the adhesive state, controlling adhesion and detachment like a "switch."
Electric response interface: After applying voltage, the charge distribution at the interface changes, thereby altering the interface energy or adsorption behavior, which can be used for interfacial force regulation or to drive behavior in materials.
pH-responsive interface: exhibits different hydrophilic-hydrophobic behaviors under different acidic and alkaline conditions, commonly used in drug release, biomimetic self-assembly systems.
One-sentence summary:
The interface is getting "smarter"—
From the initial "stick as soon as it's built" to the current "on-demand response"
Future interfaces will not only stick firmly, but also self-regulate, precisely control, and release functions as needed.
6. How to make the interface stronger?
This is what engineers care about the most: knowing it's important, so how do I get it done?
Common strategies to enhance interface strength:

One-sentence summary:
The key to interface design is not "stickiness," but "good collaboration."
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






