Tokyo Science University Develops Low-Temperature Hydrogen Battery to Overcome Hydrogen Storage Barriers
According to foreign media reports, researchers at the Institute of Science Tokyo in Japan have developed a hydrogen battery that operates at a temperature of only 90°C, overcoming the limitations of high temperature and low capacity associated with previous methods.
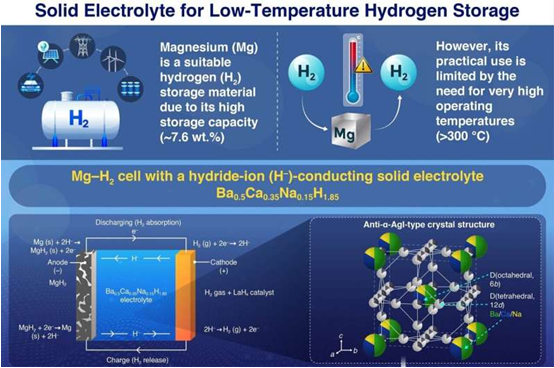
Image source: Tokyo University of Science
The working principle of this device is the movement of hydrogen anions in a solid electrolyte, allowing magnesium hydride, as the anode, to repeatedly store and release its full capacity of hydrogen. This battery provides a practical method for hydrogen fuel storage, paving the way for hydrogen-powered vehicles and clean energy systems.
One of the most pressing challenges facing hydrogen use is its storage, which often requires very low temperatures (-252.8°C) and high pressures (350 to 700 bar). A more effective approach than storing hydrogen in gas or liquid form is to store it in solid materials with high theoretical storage capacities, such as magnesium hydride (MgH2).
This material can be integrated into battery-like systems, where not only mobile electrons, but also hydrogen itself can be stored and released during the charging and discharging processes.
Until recently, this method was still limited by the following: the need for high operating temperatures above 300°C, poor reversibility of hydrogen absorption and desorption, and adverse side reactions that reduce performance.
The relevant paper of this study was published in the journal "Science," and was completed by the research team led by Dr. Takashi Hirose, Associate Professor Naoki Matsui, and Professor Ryoji Kanno from the Research Center for All-Solid-State Battery, Institute of Science, Tokyo.
Matsui stated, "We demonstrated the performance of Mg–H2 batteries as safe and efficient hydrogen energy storage devices, achieving high capacity, low temperature, and reversible hydrogen absorption and release."
The innovation of this battery lies in its solid electrolyte Ba0.5Ca0.35Na0.15H1.85, which can efficiently transport hydrogen ions, especially hydride ions (H-). This material has a reverse α-AgI-type crystal structure, renowned for its exceptionally high ionic conductivity. In this structure, barium, calcium, and sodium occupy the body-centered positions, while H- moves through face-sharing tetrahedral and octahedral interstitial sites, enabling free migration. Tests indicate that this material has a high ionic conductivity (2.1 × 10^-5 S cm^-1) and electrochemical stability at room temperature, allowing the system to effectively store and release hydrogen gas over an extended period.
The battery design uses MgH2 as the anode and hydrogen gas (H2) as the cathode. During charging, MgH2 releases H–, which migrates through the Ba0.5Ca0.35Na0.15H1.85 electrolyte to the H2 cathode, where it is oxidized to release H2 gas. During discharging, the opposite occurs: H2 gas at the cathode is reduced to H–, which migrates through the electrolyte to the anode and reacts with Mg to form MgH2. This process allows the battery to store and release hydrogen gas as needed, with all processes occurring at controlled temperatures below 100°C. Using this battery, researchers were able to achieve the full theoretical storage capacity of MgH2 in repeated cycles, which is approximately 2,030 mAh g-1, equivalent to 7.6 wt.% (weight percent) of hydrogen gas.
Traditional solid-state hydrogen storage methods face significant limitations. The thermally driven absorption and desorption process requires extremely high temperatures of 300 to 400°C to achieve hydrogen release or capture, making the process energy-intensive and unsuitable for everyday applications. Another method uses liquid electrolytes for electrochemical hydrogen storage at lower temperatures, but the hydrogen ion transport performance is poor, meaning these materials cannot achieve levels close to their theoretical hydrogen storage capacity. Therefore, neither method has provided an efficient, reversible, and low-temperature hydrogen storage solution.
Hirose explained, "These characteristics of our hydrogen storage batteries were previously unattainable by traditional thermal methods or liquid electrolytes, laying the foundation for constructing an efficient hydrogen storage system suitable as a hydrogen energy carrier."
This type of battery could be key to the future of hydrogen power, driving the development of hydrogen-powered vehicles and zero-carbon emission industries.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






