Abstract:To address the shortcomings of acrylonitrile-butadiene-styrene (ABS) plastics, such as flammability and high smoke production, as well as the problems of high addition levels and deterioration of mechanical properties when using intumescent flame retardants (IFR) alone, a liquid-phase exfoliation method is proposed to prepare graphite-phase C.3N4A nanosheet (CN) was compounded with IFR (a mixture of ammonium polyphosphate, dipentaerythritol, and melamine in a mass ratio of 2:1:1) to construct a synergistic flame retardant system, and ABS/CN/IFR composites were prepared via melt blending. The microstructure of CN and ABS/CN/IFR composites was characterized using Fourier Transform Infrared Spectroscopy, X-ray Photoelectron Spectroscopy, and Scanning/Transmission Electron Microscopy. The results showed that CN retained its triazine ring structure with uniform distribution of C and N elements, and that CN and IFR were evenly dispersed in the ABS matrix. It was found that the composite material with CN and IFR mass fractions of 1% and 20% respectively (ABS/CN1/IFR20) had a tensile strength of 67.4 MPa, which was a 15.2% increase compared to pure ABS (58.5 MPa), and higher than composites with IFR or CN alone (61.4, 64.2 MPa). The maximum thermal decomposition temperature of ABS/CN1/IFR20 composites increased from 417℃ for pure ABS to 435℃, while the peak heat release rate and total smoke release decreased by 45.2% and 38.5% respectively compared to ABS. The char residue rate increased from 1.47% for pure ABS to 15.45%. Char residue analysis revealed the formation of a continuous, dense, and relatively smooth surface char layer. The IFR-CN flame retardant system successfully addressed the challenge of balancing flame retardant efficiency and mechanical properties by leveraging the physical barrier and catalytic charring effect of CN in synergy with the expansion effect of IFR, while reducing the amount of flame retardant used.
Keywords:Intumescent flame retardant; C3N4Nanosheets; acrylonitrile-butadiene-styrene (ABS) plastic composites; synergistic flame retardant mechanism; thermal stability; mechanical properties
Acrylonitrile-butadiene-styrene (ABS) plastic is widely used in electronics, automotive, and construction fields due to its excellent mechanical properties and processability. However, the flammability of ABS and the release of toxic smoke during combustion pose serious safety risks. Therefore, the development of highly efficient flame-retardant ABS composites has become a research hotspot.[1-2]Traditional flame retardants (such as halogenated compounds) can inhibit combustion, but they have issues such as deterioration of mechanical properties and environmental toxicity. In recent years, intumescent flame retardants (IFR) have attracted attention for their low smoke and halogen-free characteristics.[3-6]However, its standalone use often faces challenges in balancing insufficient flame retardant efficiency and mechanical properties.[7]Meanwhile, two-dimensional nanomaterials (such as graphene, MoS2Due to its unique physical barrier effect[8-10]It is used to synergistically enhance the flame retardancy and mechanical properties of composites, but its high cost and complex preparation process limit practical applications.
Graphitic carbon nitride (g-C)3N4As a new type of non-metallic nanomaterial, it exhibits high thermal stability, chemical inertness, and an abundance of nitrogen elements, showing potential in the fields of catalysis and flame retardancy. Research indicates that g-C...3N4Nanosheets (CN) can delay the diffusion of pyrolysis gases through a physical barrier effect and catalyze the carbonization of the matrix, but their individual use has limited improvement on the flame retardant performance of polymers.[11-13]Therefore, combining CN with intumescent flame retardants (IFR)[13-15]By constructing a synergistic flame retardant system, it is expected to reduce the amount of flame retardant used while simultaneously enhancing mechanical properties.[16-17]However, existing studies still lack a systematic investigation into the preparation process of CN, its dispersion in polymers, and its synergistic mechanism with IFR.
The author prepared highly dispersed CN via liquid-phase exfoliation and compounded it with IFR into an ABS matrix, aiming to develop ABS composites with high flame retardancy, excellent mechanical properties, and low smoke emission. Through systematic characterization of the structural evolution of CN, the thermal stability, combustion behavior, and char residue characteristics of the composites were analyzed, elucidating the synergistic flame-retardant mechanism between CN and IFR, thus providing a theoretical basis for the design and application of high-performance flame-retardant polymers.
ABS: DG-417, Tianjin Dagu Chemical Co., Ltd.
Urea: U111897, purity ≥99%, Shanghai Aladdin Biochemical Technology Co., Ltd.
Sulfuric acid: 10021608, purity 95%~98%, Shanghai Chemical Reagent Co., Ltd.;
Ammonium Polyphosphate (APP): A189185n≥1,000, Shanghai Aladdin Biochemical Technology Co., Ltd.
Melamine (MEL): M108433, purity ≥99%, Shanghai Aladdin Biochemical Technology Co., Ltd.
Dipentaerythritol (DPER): D101402, purity ≥90%, Shanghai Aladdin Biochemical Technology Co., Ltd.
1.2 Main Instruments and Equipment
Fourier Transform Infrared Spectrometer (FTIR): Nicolet-5700, Shanghai Lairui Scientific Instrument Co., Ltd.
Scanning Electron Microscope (SEM): S-4800 (Ⅱ), Hitachi Ltd., Japan;
X-ray diffraction (XRD) instrument: Bruker AXS D8, Bruker Corporation, Germany;
X-ray Photoelectron Spectroscopy (XPS) instrument: Kratos AXIS Ultra DL, Shimadzu Corporation, Japan.
Transmission Electron Microscope (TEM): JEM 2100, JEOL Ltd., Japan;
Energy Dispersive X-ray Spectroscopy (EDX) Instrument: X-MaxN 100TLE, Oxford Instruments Group, UK;
Thermogravimetric Analyzer (TG): TGA-601, Nanjing Huicheng Instrument and Meter Co., Ltd.
Cone Calorimeter: ZY-6243, Dongguan Zhongnuo Quality Inspection Instrument Equipment Co., Ltd.
Electronic Universal Testing Machine: AG-IS, Shimadzu Corporation, Japan.
Mini twin-screw extruder: SJZS-10A, Wuhan Ruiming Experimental Instruments Co., Ltd.
Micro injection molding machine: SZS-20, Wuhan Ruiming Experimental Instrument Co., Ltd.
Granulator: SY-6217-ZBQ-20, Dongguan Shiyan Precision Instruments Co., Ltd.;
Program-controlled tablet press: LP-S-50, Shanghai Langtai Engineering Co., Ltd.
1.3.1 Preparation and Exfoliation Treatment of Bulk CN (B-CN)
Preparation of B-CN: Weigh 10 g of urea and place it in a muffle furnace. Heat it to 430 °C at a rate of 5 °C/min and react for 4 hours. Then, increase the temperature to 520 °C and continue reacting for 2 hours. After cooling to room temperature, a pale yellow product, namely B-CN, is obtained.
B-CN exfoliation treatment: Using the liquid-phase exfoliation method, B-CN is placed in a 25% sulfuric acid solution and stirred for 48 hours to obtain a pale yellow suspension. The suspension is evenly distributed into 6 centrifuge tubes and centrifuged at a speed of 8,000 r/min for 5 minutes. Once centrifugation is complete, remove the centrifuge tubes and discard the supernatant. Then add 15 mL of deionized water and centrifuge again, repeating the above steps until the supernatant is neutral. Finally, the solid product is dried in a vacuum drying oven at 80°C for 24 hours to obtain CN.
1.3.2 Preparation of ABS/CN/IFR Composites
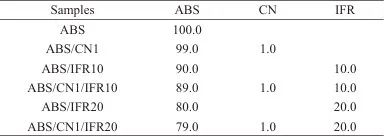
Mix and grind APP, DPER, and MEL in a mass ratio of 2:1:1 to obtain IFR, then dry it at 80°C for 24 hours. Pre-mix ABS, IFR, and CN in a certain ratio, and use a mini twin-screw extruder for melt blending. Set the extruder temperatures at 170, 190, and 200°C for the three zones, with a screw speed of 50 r/min. Use a pelletizer to produce pellets, setting the pelletizer speed to 50 r/min, to obtain ABS/CN/IFR composites. See Table 1 for the specific formulation.
Table 1 Formulation of ABS/CN/IFR composites (mass fraction) (%)
1.4 Testing and Characterization
1.4.1 FTIR Characterization
Dry the test sample in an oven at 80°C for 12 hours, then prepare a KBr pellet and measure in the range of 400-4000 cm.-1FTIR characterization was performed within the above-mentioned wavenumber range.
1.4.2 SEM Characterization
Take a small amount of the dried sample and place it on the surface of conductive adhesive for gold sputtering treatment. Then, send it to the SEM observation chamber to observe and photograph the sample morphology.
1.4.3 XRD Characterization
Using an XRD instrument for testing, the scan employs a Cu target with an incident wavelength of 0.154 nm. The operating voltage and current are 40 kV and 40 mA, respectively, with a scanning range of 2° to 40°.
1.4.4 XPS Characterization
Using XPS to study the morphology and chemical composition of the sample, with an excitation wavelength of 0.84 nm.
Dry the test sample as a pretreatment, then weigh 3~10 mg of the sample and place it in a clean, dry crucible. Use a TG analyzer under N2Under an atmosphere, heat from 30 ℃ to 800 ℃ at a rate of 10 ℃/min to conduct the test, and record the TG curve and DTG curve.
1.4.6 Cone Calorimeter Test
Dry the test sample, then use a program-controlled tablet press to prepare the test sample with dimensions of 100 mm × 100 mm × 3 mm. Test according to ISO 5660-1, with the sample wrapped in aluminum foil and placed horizontally in the cone calorimeter, with a heat flux of 35 kW/m².2Three parallel specimens are prepared for each group of samples. The test results are averaged, and the error originates from the standard deviation of three independent tests.
1.4.7 TEM Characterization
Composite samples were prepared using cryo-ultramicrotomy for TEM observation. After freezing, the sample was gently scraped with a blade to obtain sections with a thickness less than 100 nm. The samples were placed in small tubes, immersed in anhydrous ethanol, and ultrasonicated for 10 minutes. After drying, the samples were observed and EDX mapping analysis was performed.
1.4.8 Tensile Performance Test
According to GB/T 1040.2-2022, prepare dumbbell-shaped specimens of ABS and its composites. Use an electronic universal testing machine to test their tensile properties. The sample dimensions are 75 mm × 5 mm × 2 mm, with a tensile rate of 100 mm/min. Prepare 5 standard dumbbell-shaped specimens for each formulation group, and exclude abnormal values where the fracture location is more than 2 mm from the gauge marks.
2.1 CN Structure Characterization
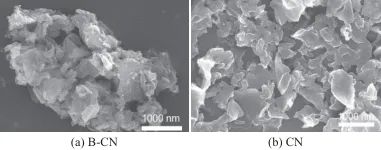
Figure 1 shows SEM images of B-CN and CN. As shown in Figure 1a, the unexfoliated B-CN exhibits a highly aggregated layered structure with some blocky agglomerates, with an overall size of about 5 μm. As shown in Figure 1b, after liquid-phase exfoliation, the obtained CN presents a clearer and more distinct sheet-like structure, with the sheets uniformly dispersed and sized between 100 nm and 2 μm.
Fig. 1 SEM images of B-CN and CN
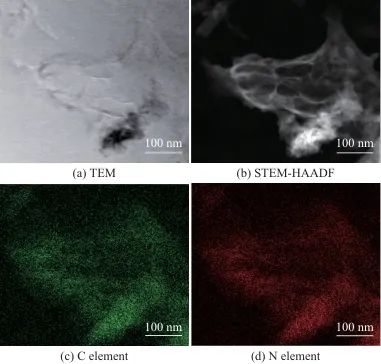
Figure 2 shows the TEM image of CN, the scanning transmission electron microscopy-high-angle annular dark field (STEM-HAADF) image, and the corresponding C and N elemental distributions. As shown in Figure 2a, CN exhibits a typical slightly wrinkled sheet-like structure, indicating the successful synthesis of CN. Figures 2b, 2c, and 2d show the HAADF image of CN and the corresponding C and N elemental maps of the same area, respectively. It can be seen that the C and N elements are uniformly distributed in the synthesized sample, further confirming the successful preparation of CN.
Fig. 2 TEM and STEM-HAADF images and the corresponding elemental mappings of CN
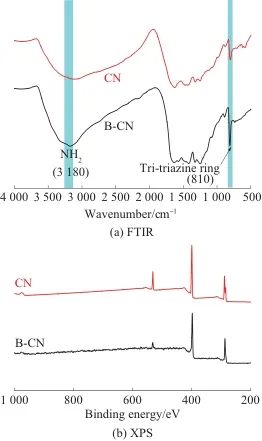
Figure 3 shows the FTIR and XPS spectra of B-CN and CN. As shown in Figure 3a, B-CN and CN exhibit peaks at 3180 and 810 cm⁻¹.-1The characteristic absorption peaks are shown, corresponding to amino and triazine ring structures; in the range of 1200~1700 cm.-1Continuous characteristic peaks appeared in the range, corresponding to the C=N and C—N stretching vibrations on the CN heterocycle, as well as the C—N stretching vibration outside the ring.[18]The FTIR spectra of B-CN and CN are basically consistent, indicating that liquid-phase exfoliation has not caused significant changes to the functional groups in CN. As shown in Figure 3b, signals of C, N, and O elements are detected in the XPS survey spectra of both B-CN and CN, and the peak shapes are essentially the same. The mass percentages of C, N, and O in B-CN are 43.51%, 52.07%, and 4.42%, respectively, while those in CN are 41.65%, 49.31%, and 9.04%, respectively.
Fig. 3 FTIR and XPS spectra of B-CN and CN
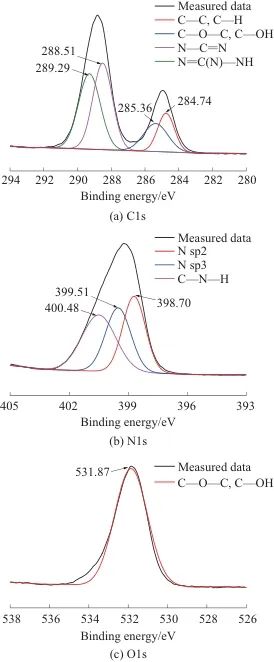
XPS was used to perform a more detailed analysis of the surface elemental composition and chemical state of the CN obtained by liquid-phase exfoliation, in order to confirm its structural characteristics and evaluate its surface state. Figure 4 shows the C 1s, N 1s, and O 1s spectra of CN. As shown in Figure 4a, the C 1s spectrum of CN exhibits four peaks: the peak at 284.74 eV corresponds to C—C and C—H, the peak at 285.36 eV is attributed to C—O—C and C—OH, and the peaks at 288.51 eV and 289.29 eV correspond to N—C=N and N=C(N)—NH, respectively, indicating the presence of CN. As shown in Figure 4b, the N 1s spectrum shows three peaks at 398.70, 399.51, and 400.48 eV, which correspond to C—N=C, N—(C), respectively.3,C—N—H[19]The O element signal mainly appears at 531.87 eV, corresponding to the peaks of C—O—C and C—OH (Figure 4c). The abundant nitrogen element, the catalytic/gas source role of terminal amino groups, and the potential reactivity of oxygen-containing groups provide a key basis for subsequently explaining its excellent flame retardant and smoke suppression performance.
Fig. 4 Fine XPS spectrum of CN
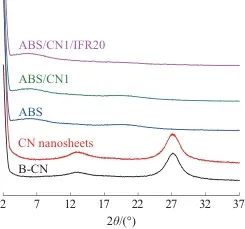
Figure 5 shows the TEM image of ABS/CN1/IFR20. As shown in the figure, the flame retardant is uniformly distributed in the ABS matrix, with particle diameters ranging from 5 to 10 μm. Figure 6 presents the XRD patterns of B-CN, CN, and ABS composites. B-CN and CN exhibit two distinct diffraction peaks at approximately 13.1° and 27.4°, which correspond to the (100) and (002) crystal planes of CN, respectively. These peaks are attributed to the in-plane stacking of triazine ring structural units and interlayer stacking of the conjugated aromatic system in CN. The peak shapes of B-CN and CN are basically consistent, indicating that liquid-phase exfoliation does not significantly affect the crystal structure of CN. No characteristic diffraction peaks of CN are observed in the XRD pattern of ABS, nor in those of the ABS/CN1 and ABS/CN1/IFR20 composites, indicating that CN has lost its structural regularity. In addition, no characteristic diffraction peaks corresponding to APP, MEL, or DPER are observed in the XRD patterns, suggesting that the IFR undergoes melting and its crystal structure is destroyed during processing, resulting in a high degree of dispersion. The above results demonstrate that both CN and IFR can be uniformly distributed in the ABS matrix.
Fig. 5 TEM images of ABS/CN1/IFR20
Fig. 6 XRD patterns of B-CN, CN, ABS, and their composite materials
2.2 Mechanical Properties of ABS/CN/IFR Composites
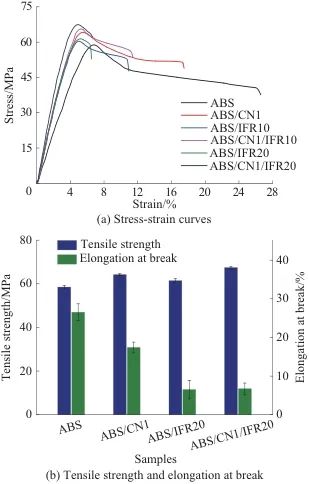
Figure 7 shows the stress-strain curves (Figure 7a) and the comparison of mechanical properties (Figure 7b) for ABS/CN/IFR composites. As shown in Figure 7, the tensile strengths of ABS, ABS/IFR10, and ABS/IFR20 are 58.5, 60.3, and 61.4 MPa, respectively, and the elongations at break are 26.5%, 10.9%, and 6.5%, respectively. With the increase in IFR content, the tensile strength of ABS does not change significantly, but the elongation at break decreases significantly, with a maximum reduction of 75.5% compared to ABS. The tensile strength of ABS/CN1 is 64.2 MPa, and the elongation at break is 17.4%. The tensile strength of ABS/CN1 is enhanced by 9.7% compared to ABS, indicating that adding 1% by mass of CN can enhance the tensile strength of ABS. The tensile strength of ABS/CN1/IFR20 is 67.4 MPa, representing an increase of 5.0% and 9.8% compared to ABS/CN1 and ABS/IFR20, respectively, indicating that CN and IFR can have a synergistic effect to jointly improve the tensile strength of ABS.
Fig. 7 Tensile properties of ABS and its composite materials
2.3 Thermal Stability of ABS/CN/IFR Composite Materials
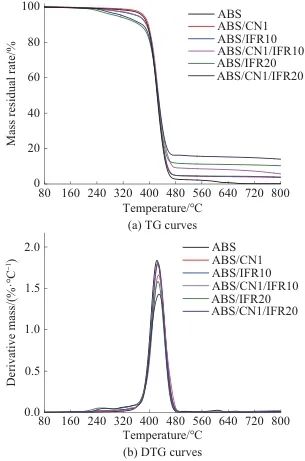
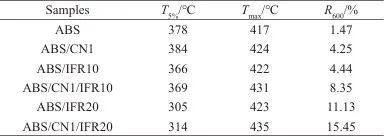
Figure 8 shows the TG and DTG curves of ABS and its composites, with detailed data shown in Table 2. As illustrated in Figure 8, ABS and its composites degrade in a single step. The initial decomposition temperature of ABS (T5%) was 378 ℃, and for ABS/IFR10 and ABS/IFR20T5%They are 366 ℃ and 305 ℃ respectively, indicating that with the increase of IFR, the ABS'sT5%showed a downward trend, with a maximum decrease of 73℃. This is mainly due to the instability of the IFR at high temperatures, which leads to the premature decomposition of the composites. Compared to pure ABS, ABS/CN1’sT5%Increased to 384°C, indicating that CN can improve the thermal stability of ABS. As shown in Figure 8b, the maximum thermal decomposition temperature of ABS (Tmax) is 417°C, ABS/CN1, ABS/IFR20, and ABS/CN1/IFR20Tmax
They are 424°C, 423°C, and 435°C, respectively. Compared to ABS resin, ABS/CN1/IFR20'sTmaxThe increase of 4.3% may be due to the physical barrier effect of CN on the thermal decomposition of ABS and the expansion and carbonization effect of IFR. As shown in Table 2, the char yield of ABS at 600 ℃R600The content is only 1.47%; for ABS/IFR10 and ABS/IFR20R600The values are 4.44% and 11.13%, respectively, indicating that as the amount of IFR added increases, the ABS's...R600Gradually increasing. The ABS/CN1’sR6004.25%; ABS/CN1/IFR20R600The maximum reached 15.45%, an increase of 13.98% compared to ABS. In summary, CN and IFR can jointly improve the thermal stability and char-forming ability of ABS. Good char-forming ability usually helps to inhibit the release of heat and combustible gases, thereby enhancing the flame retardancy of the composite material.
Fig. 8 Thermal stability of ABS and its composite materials
Table 2 Thermal stability analysis data of ABS and its composite materials
Notes:T5% is the temperature at 5% mass loss;Tmax is the temperature at maximum mass loss;R600 is residual carbon rate at 600 ℃.
2.4 Flame Retardant Properties of ABS/CN/IFR Composite Materials
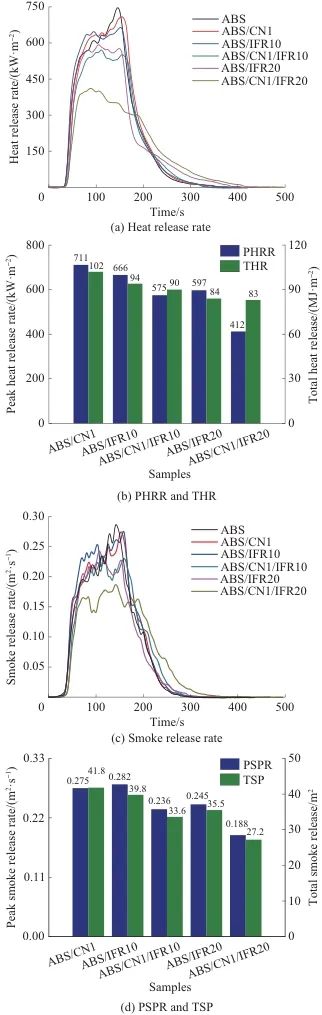
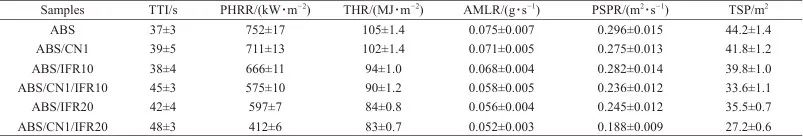
Figure 9 shows the heat release rate curve, peak heat release rate (PHRR), total heat release (THR), smoke release rate curve, peak smoke release rate (PSPR), and total smoke production (TSP) of ABS and its composites. Detailed data can be found in Table 3. The PHRR and THR of ABS are 752 kW/m.2105 MJ/m2As shown in Figures 9a and 9b, the PHRR values of ABS/IFR10 and ABS/IFR20 are 666 and 597 kW/m², respectively.2, THR decreased to 94 and 84 MJ/m respectively2The above results indicate that IFR can effectively reduce the PHRR and THR of ABS, and both PHRR and THR of ABS gradually decrease with increasing IFR content. The PHRR and THR of ABS/CN1 are reduced by 5.5% and 3.0%, respectively, compared to ABS resin, indicating that CN has a certain inhibitory effect on the PHRR and THR of ABS. The PHRR and THR of ABS/CN1/IFR20 are 412 kW/m² and ...2and 83 MJ/m2Compared to pure ABS, it decreased by 45.2% and 21.0%. The reduction in PHRR and THR of ABS composites is mainly due to the physical barrier effect of CN and the intumescent charring action of IFR. The addition of CN and IFR can effectively extend the time to ignition (TTI) of ABS resin. The TTI for pure ABS is 37 seconds, while the TTI for ABS/CN1/IFR10 and ABS/CN1/IFR20 is extended to 45 seconds and 48 seconds, respectively. AMLR refers to the average mass loss rate of composites during combustion, and the AMLR of ABS/CN1/IFR20 is reduced by 30.7% compared to pure ABS.
Fig. 9 Combustion performance of ABS and its composite materials
Table 3 Cone calorimetric test data of ABS/CN/IFR composites
Notes:TTI is ignition time;PHRR is peak heat release rate;THR is total heat release;AMLR is average mass loss rate;PSPR is peak smoke release rate;TSP is total smoke release.
Thick smoke is one of the key factors leading to casualties in fires. As shown in Figures 9c and 9d, the PSPR and TSP of pure ABS are 0.296 m.2/s and 44.2 m2The PSPR of ABS/IFR10 and ABS/IFR20 decreased to 0.282 and 0.245 m, respectively.2/s, TSP are 39.8 and 35.5 m, respectively.2It indicates that IFR can reduce the PSPR and TSP of ABS during the combustion process, and the reduction effect becomes more significant with the increase of IFR content. The PSPR and TSP of ABS/CN1 are 0.275 m2/s and 41.8 m2The addition of CN with a mass fraction of only 1% has little effect on the PSPR and TSP of ABS resin. When 1% CN and 20% IFR are added simultaneously, there is a significant reduction in the PSPR and TSP of ABS. The PSPR of ABS/CN1/IFR20 is reduced by 36.5%, and the TSP is reduced by 38.5% compared to ABS. The above results indicate that CN and IFR can effectively reduce the heat and smoke produced during the combustion process of ABS.
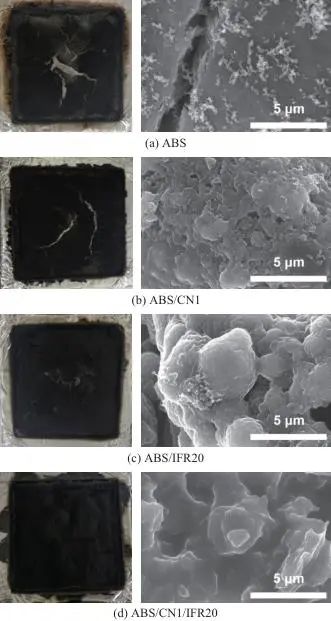
Figure 10 shows digital photographs and SEM images of the char residues of ABS and its composites, with EDX data listed in Table 4. From the analysis of the digital photographs, it is evident that ABS resin burns almost completely, leaving only a small amount of char. In contrast, after adding CN or IFR (ABS/CN1 and ABS/IFR20), the amount of char residue in the composites significantly increases, with a thicker char layer and fewer cracks. Particularly, the surface of the ABS/IFR20 char residue exhibits an expanded structure. The char residue of the ABS/CN1/IFR20 composite is macroscopically the most continuous and dense, with the thickest char layer and the most prominent expanded structure, which helps to block the release of heat and smoke. Further observation of the microstructure of the char residues through SEM reveals that the surface of the ABS char residue is covered with fine cracks and holes. The ABS/CN1 char residue increases in amount, and its microstructure shows a large number of CNs interconnected, forming a dense barrier that enhances the strength and continuity of the char residue. The ABS/IFR20 char residue shows an expanded structure on a microscopic scale, with a relatively smooth surface. The ABS/CN1/IFR20 char residue features both an expanded structure and a large amount of CN tightly attached to the char surface, giving its microstructure a high degree of continuity and density. The significant improvement in the flame retardant and smoke suppression performance of the composites is mainly attributed to the synergistic effects of the physical barrier effect of CN and the intumescent charring action of IFR, which together contribute to the formation of a dense, continuous, and expanded char layer, effectively hindering the transfer of heat and oxygen.
Fig. 10 Digital photos (left) and SEM images (right) of carbon residue after cone calorimetry test
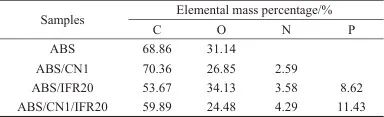
Table 4 EDX Data of ABS and Its Composite Carbon Residues
Table 4 shows the EDX data of char residues from ABS and its composites. In the char residue of ABS, the mass fractions of C and O elements are 68.86% and 31.14%, respectively. When 1 wt% CN is added, the mass fraction of C in the ABS/CN1 char residue increases to 70.36%, indicating that CN enhances the carbonization ability of the ABS composite. In the ABS/IFR20 char residue, N and P elements are sufficiently detected, with mass fractions of 3.58% and 8.62%, respectively, indicating that IFR is mainly retained in the char residue. Compared with ABS/IFR20, the mass fraction of C in the ABS/CN1/IFR20 char residue increases by 6.22%, and the mass fractions of N and P elements also show certain improvements, further demonstrating that the addition of CN can enhance the carbonization ability of the ABS composite. In summary, CN and IFR can promote the intumescent charring of ABS, thereby improving the flame retardant performance of the ABS composite.
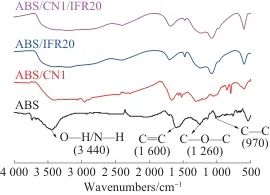
Figure 11 shows the FTIR spectra of the residues of ABS, ABS/CN1, ABS/IFR20, and ABS/CN1/IFR20. The residue of ABS at 3,440 cm.-1A relatively broad peak appears here, corresponding to the stretching vibrations of amino and hydroxyl groups, at 1600, 1260, and 970 cm⁻¹.-1Characteristic absorption peaks are displayed at the respective positions, corresponding to the C=C, C—O—C, and C—C groups. Compared with pure ABS, ABS/CN1 shows at 810 cm⁻¹.-1A distinct characteristic peak appears on both sides, corresponding to the triazine ring structure of CN, indicating that CN mainly exists in the char residue. In the char residues of ABS/IFR20 and ABS/CN1/IFR20, within the range of 850~1100 cm⁻¹...-1The observed peak corresponds to the P—O—P stretching vibration absorption peak. In summary, the main components in the char residue of the ABS composite after combustion are CN and IFR. During the combustion process, as the high-temperature degradation of ABS continues, CN gradually migrates to the surface of the composite, while IFR can promote the expansion and charring of ABS, forming a continuous and dense intumescent char layer. This layer serves as a barrier against oxygen and heat, thereby achieving a flame-retardant effect.
Fig. 11 FTIR spectra of residues
3 Conclusion
(1) B-CN was prepared by thermal polymerization of urea and graphite-phase CN was obtained through liquid-phase exfoliation. After mixing it with IFR, ABS/CN/IFR composites were prepared by melt blending with ABS. TEM and XRD results indicate that CN and IFR are well dispersed in ABS, and their synergistic effect significantly enhances the mechanical properties of the material. The ABS/CN1/IFR20 composite with 1 wt% CN and 20 wt% IFR exhibits a tensile strength of 67.4 MPa, outperforming pure ABS at 58.5 MPa.
Thermogravimetric analysis indicates that the early decomposition of IFR leads to the composite materialT5%decreased, but the synergy between CN and IFR significantly delayed the high-temperature decomposition process.TmaxThe temperature increased to 435 ℃ (an 18 ℃ improvement compared to pure ABS), and the char residue rate increased to 15.45%, demonstrating that the system has excellent thermal stability and a char-forming barrier effect at high temperatures. Cone calorimeter test results show that compared to pure ABS, the ABS/CN1/IFR20 composite material exhibits significant reductions in PHRR, THR, PSPR, and TSP, along with an extended TTI.
The char residues of ABS composites were tested and characterized. The results showed that the addition of CN and IFR made the char layer denser and more continuous, and there was a distinct intumescent structure. This structure is beneficial for suppressing the release of heat and smoke, thereby improving the flame retardancy and smoke suppression performance of ABS.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.