MIT Invents Simple Formula That Could Guide Faster Charging and Longer-Lasting Battery Design
The core of all lithium-ion batteries is a simple reaction: during the discharge of the battery, lithium ions dissolved in the electrolyte solution "embed" into the solid electrode. When they are extracted and return to the electrolyte, the battery charges.
This process occurs thousands of times during the entire lifespan of a battery. The power output and charging speed of the battery depend on the speed of this reaction. However, little is known about the exact mechanism of this reaction and the factors that control its rate.
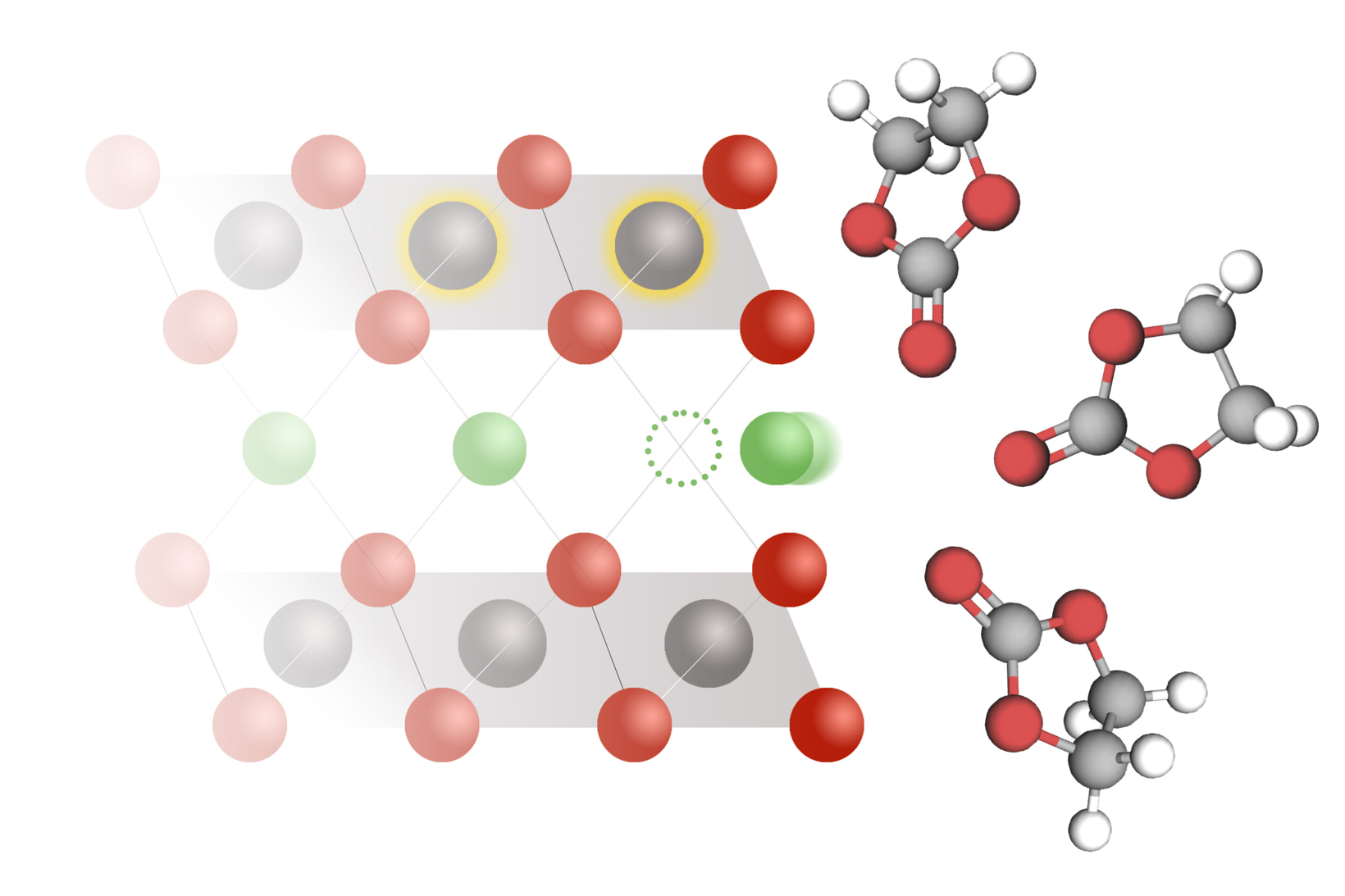
Image source: Journal "Science"
According to foreign media reports, in a study published in the journal "Science," researchers from the Massachusetts Institute of Technology (MIT) measured the lithium intercalation rates in various battery materials and used this data to develop a new model to control the reaction. The model indicates that lithium intercalation is governed by a process known as coupled ion-electron transfer, in which electrons are transferred to the electrode along with lithium ions.
Researchers say that the insights gained from this model can guide the design of more robust and faster-charging lithium-ion batteries. "We hope that through this study, we can make the reactions faster and more controllable, thereby accelerating the charging and discharging speed," said Martin Bazant, Chevron Professor of Chemical Engineering and Professor of Mathematics at MIT.
The new model may also help scientists understand why adjusting the electrodes and electrolytes in certain ways can improve energy, power, and battery life— a process that has primarily been achieved through trial and error.
"In this paper, we begin to unify the observed reaction rates of different materials and interfaces into a coupled electron and ion transfer intercalation theory, thereby consolidating previous research findings on reaction rates," said Yang Shao-Horn, Professor of Engineering at MIT and Professor of Mechanical Engineering, Materials Science and Engineering, and Chemistry.
Lithium flow modeling
For decades, scientists have assumed that the insertion rate of lithium ions into the electrodes of lithium-ion batteries depends on the speed at which lithium ions diffuse from the electrolyte to the electrode. They believe that this reaction is governed by the Butler-Volmer equation, a model originally developed nearly a century ago to describe the charge transfer rate in electrochemical reactions.
However, when researchers attempt to measure the rate of lithium intercalation, the measured results do not always align with the rates predicted by the Butler-Volmer equation.
Furthermore, obtaining consistent measurement results between different laboratories has always been challenging, with the measurement results of the same reaction reported by different research teams differing by as much as a billion times.
In this new study, the research team at MIT used an electrochemical technique to measure the lithium insertion rate, which involves applying repeated short voltage pulses to the electrode.
Researchers conducted these measurements on more than 50 combinations of electrolytes and electrodes, including lithium nickel manganese cobalt oxide commonly used in electric vehicle batteries, as well as lithium cobalt oxide used in most smartphones, laptops, and other portable electronic devices.
The measured rates for these materials are much lower than previously reported and do not match the predictions of the traditional Butler-Volmer model.
Researchers proposed another theory regarding the mechanism of lithium embedding at the electrode surface based on these data. This theory is based on the assumption that in order for lithium ions to enter the electrode, electrons from the electrolyte solution must simultaneously transfer to the electrode.
"The electrochemical step is not the insertion of lithium (which might be considered the main step), but rather the transfer of electrons, with the aim of reducing the solid material that carries lithium," Bazant said. "The insertion of lithium and the transfer of electrons occur simultaneously and promote each other."
This kind of Coupled Ionic Electron Transfer (CIET) reduces the energy barrier that must be overcome for intercalation reactions to occur, making them more likely to happen. The mathematical framework of CIET enables researchers to predict reaction rates, which have been validated by experiments and differ significantly from the predictions of the Butler-Volmer model.
Faster charging speed
In this study, the researchers also demonstrated that they could adjust the insertion rate by altering the composition of the electrolyte. For instance, swapping different anions can reduce the energy required for transferring lithium and electrons, thereby increasing the efficiency of the process.
Shao-Horn stated, "By altering the electrolyte to regulate the embedding kinetics, there is a great opportunity to enhance the reaction rate and modify the electrode design, thereby improving the battery's power and energy."
Shao-Horn's lab and its collaborators have been using automated experiments to create and test thousands of different electrolytes, which are used to develop machine learning models to predict functionally enhanced electrolytes.
These findings can also help researchers design batteries with faster charging speeds by accelerating the lithium intercalation reaction. Another goal is to reduce side reactions that may lead to battery performance degradation when electrons detach from the electrode and dissolve into the electrolyte.
"If you want to do this rationally, rather than just through trial and error, you need some theoretical framework to understand which important material parameters you can adjust," Bazant said. "That's exactly what this paper aims to provide."
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






