Guiyang University: Preparation and Structural Characterization of Ammonium Phytate-Coated Microcrystalline Cellulose
Abstract: Using biobased microcrystalline cellulose (MCC) as the raw material, MCC was coated and modified by gradually adding melamine (MEL) and phytic acid (PA) to prepare a biobased hybrid structure of phytate amine-coated microcrystalline cellulose (MEL-PA-MCC). The structure, elemental composition, and morphology of MEL-PA-MCC were characterized using Fourier transform infrared spectroscopy, thermogravimetric analysis, differential scanning calorimetry, scanning electron microscopy, X-ray photoelectron spectroscopy, energy dispersive spectrometry, and X-ray diffraction. The results showed that after modification with MEL-PA, MCC exhibited characteristic absorption peaks of C=N, P=O, and P—O at 1 624, 1 328, and 785 cm-1, respectively, and the maximum thermal decomposition rate significantly decreased, while the char yield increased; the surface of the MEL-PA-MCC hybrid structure was densely attached with round particles, and the N and P elements were significantly enhanced, and the crystallization behavior was also significantly changed. These results collectively indicate that the phytate amine successfully achieved the coating and modification of MCC. When MEL-PA-MCC was used to modify poly(butylene adipate-co-terephthalate) (PBAT), the composite material could achieve a UL-94 V-1 rating in vertical burning tests when the mass fraction of MEL-PA-MCC added was 5%, and the limiting oxygen index increased to 25.5%, with significant improvement in anti-dripping performance. In addition, the tensile strength and tensile elastic modulus of the PBAT/5%MEL-PA-MCC composite material were increased by 87.31% and 41.30%, respectively, compared to pure PBAT. Therefore, the introduction of the prepared MEL-PA-MCC flame-retardant hybrid structure can significantly improve the flame retardancy and tensile properties of PBAT.
keywords: phytic acid; melamine; microcrystalline cellulose; flame retardant; hybrid structure
In recent years, the preparation of hybrid flame retardants by introducing organic or inorganic particles rich in P, N flame-retardant elements for modifying composite materials has received considerable attention. Phytic acid (PA) is a phosphoric compound with high phosphorus content, featuring good biocompatibility, environmental friendliness, and certain flame-retardant properties. Under high-temperature conditions, PA decomposes to produce phosphoric acid and water, where phosphoric acid plays a role in reducing the flammability of materials [17-19]. For instance, Xiao Wencheng [18] used chitosan hydrogel as a carrier, forming a three-dimensional network structure with PA, and doped it with nanoscale silica sol to prepare flame-retardant polyester fabric. The results showed that the treated fabric had better thermal stability, with an increase of 41.5% in char yield, no melt dripping, achieving a synergistic flame-retarding effect among phosphorus, nitrogen, and silicon. Additionally, melamine (MEL), due to its high nitrogen content, low cost, and easy availability, can serve as a gas source in intumescent flame retardants, releasing a large amount of inert gases during combustion, playing an excellent synergistic flame-retarding role in the gas phase [20]. He Wenping [21] synthesized diphenylamine phosphate melamine salt (DPMS) through further reaction with MEL using o-phenylenediamine and phosphoryl trichloride as raw materials, and blended it with nylon 6 by melting. The results indicated that DPMS could significantly improve the flame-retardant performance of the nylon 6 composite, passing the UL-94 test at V-0 level. Luo Minyi [22] prepared polyphosphate (MPP) mainly from MEL and phosphoric acid, adding it to polyvinyl alcohol (PVA). When 16.6 wt% MPP was added, the PVA composite material reached a flame-retardant grade of V-0, with a significant reduction in heat release rate and a noticeable improvement in mechanical properties.
Introducing PA and MEL into the carrier, a MEL-PA hybrid flame retardant structure was prepared for modifying the polymer matrix. It is reported that Li Wenxiong [23] further reacted the prepared MEL-PA with transition metal ions Mn2+, Zn2+, Ni2+ to prepare a bio-based hybrid flame retardant for flame-retardant polypropylene. Zhang Bing et al. [24] prepared MEL-PA/RPUF composites by melting and mixing self-made MEL-PA with rigid polyurethane foam (RPUF) at a certain ratio. The study showed that compared with pure RPUF, the thermal stability of MEL-PA/RPUF composites was higher, and the residual carbon rate significantly increased to 20.6%. Wang Yiwen et al. [25] blended MEL-PA with nylon 66 by melting to prepare composites. Test results indicated that MEL-PA improved the flame retardancy of nylon 66. When the mass fraction of MEL-PA reached 8%, the UL-94 test rating of the composite could reach V-0 level, the limiting oxygen index (LOI) was greater than 27%, and the total smoke release was significantly reduced. Although both PA and MEL as raw materials for flame retardant modification have good flame retardant effects, there are few reports on the use of PA and MEL to coat and modify MCC to prepare hybrid flame retardants.
Therefore, the author uses MCC as a carrier and adopts PA and MEL to encapsulate and modify MCC, preparing phytic acid amine encapsulated MCC (MEL-PA-MCC) bio-based hybrid flame retardant material. The influence of different molar ratios of PA and MEL on the structure and composition of MCC is explored. The structure, morphology, elemental composition, and thermal stability of MEL-PA-MCC are systematically characterized. MEL-PA-MCC is applied to a biodegradable matrix - poly(butylene adipate-co-terephthalate) (PBAT), and the flame retardancy and tensile properties of PBAT/MEL-PA-MCC composites are analyzed.
1 Experimental Section
1.1 Major Raw Materials
1.2 Main Instruments and Equipment
1.3 Preparation of MEL-PA-MCC
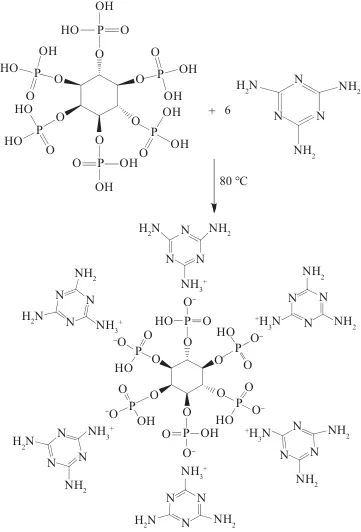
Tab. 1 Formulation of MEL-PA-MCC ( g )
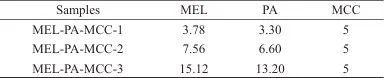
1.4 Preparation of PBAT/MEL-PA-MCC Composite Materials
Table 2 Composition ratios of different PBAT/MEL-PA-MCC composites
1.5 Testing and Characterization
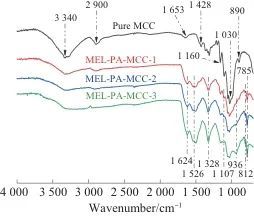
2.1 FTIR Analysis
2.2 Thermal Stability Analysis
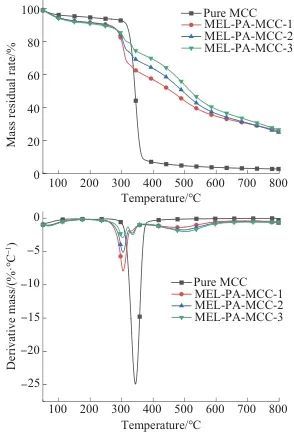
Table 3 TG and DTG data of pure MCC and MEL-PA-MCC
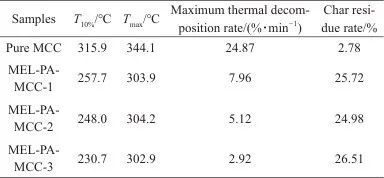
Notes:T10% is temperature of 10% weight loss;Tmax is temperature of maximum mass loss rate.
2.3 DSC Analysis
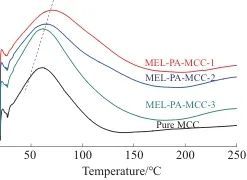
Table 4 DSC data of pure MCC and MEL-PA-MCC
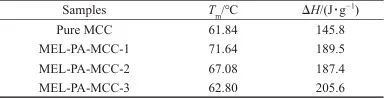
Notes:Tm is melting temperature;ΔH is enthalpy value.
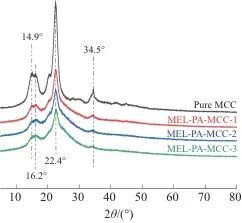
2.4 XRD Analysis
2.5 XPS Analysis
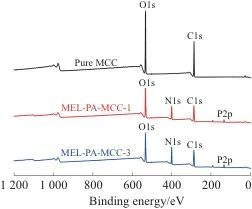
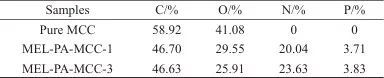
Table 5 XPS test data for pure MCC and MEL-PA-MCC
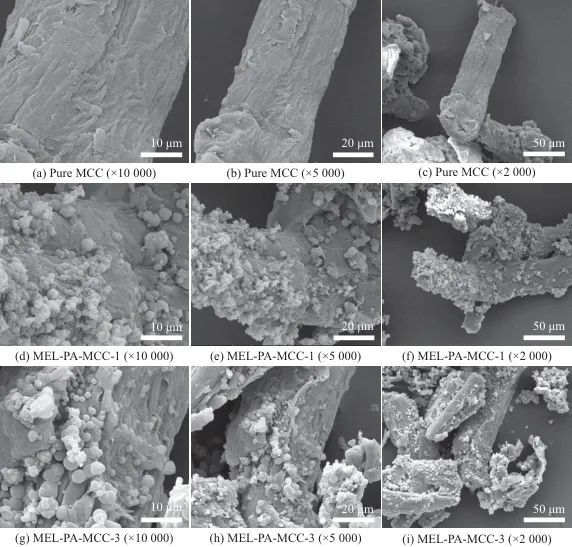
2.6 Structural Morphology Analysis
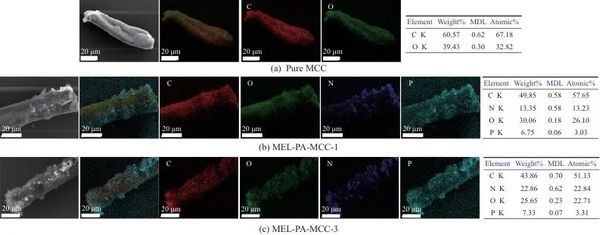
2.7 Element Composition Analysis
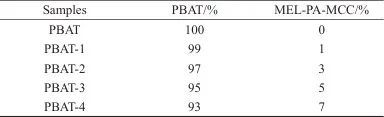
2.8 Effects of MEL-PA-MCC on the properties of PBAT/MEL-PA-MCC composites
Table 6 Flame Retardancy of Different PBAT/MEL-PA-MCC Composites
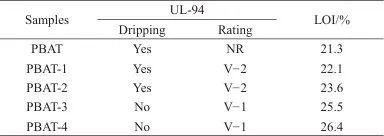
Note:NR refers to not reaching flame-retardant rating.
Using MCC as the carrier, PA and MEL were used to encapsulate and modify it, preparing a MEL-PA-MCC hybrid flame-retardant structure. The effect of adding different amounts of PA and MEL ratios on the structure, elemental composition, morphology, and thermal stability of MCC was explored. MEL-PA-MCC was introduced into PBAT resin, and its impact on the flame retardancy and tensile properties of the matrix was studied. Based on the experimental data results, the following main conclusions were obtained.
FTIR analysis indicated that MEL-PA-MCC, compared to pure MCC, mainly exhibited characteristic peaks of the triazine ring structure's C=N, N—H, P=O, and P—O at 1 624, 1 526, 1 328 cm-1, and 785 cm-1. Through SEM micro-morphology analysis, MEL-PA-MCC still had neatly arranged rod-like structures, and a large number of round particles were uniformly attached to the surface, mainly showing C, O, N, P elements uniformly adhered to the surface of MCC. The above results showed that phytic acid amine had been successfully coated on the surface of MCC.
(2) According to the thermal stability analysis, it is known that after coating modification, there is a certain degree of impact on the thermal stability of MCC. Compared with pure MCC, the maximum thermal degradation rate of MEL-PA-MCC is significantly reduced, and the char yield is significantly increased, with the highest char yield reaching 26.51%, indicating that phytic acid amine coating has significantly improved the charring ability of MCC.
(3) The crystallization behavior of phytammine-coated MCC changed significantly, with a significant reduction in diffraction peaks, indicating a decrease or weakening in crystallization behavior.
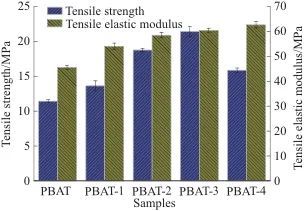
(4) The introduction of MEL-PA-MCC can effectively improve the flame retardancy and tensile properties of the PBAT matrix. When the mass fraction of MEL-PA-MCC added is 5%, the composite material reaches a flame retardant V-1 level, with LOI increased to 25.5%; compared to pure PBAT, the tensile strength and tensile elastic modulus of the PBAT/5%MEL-PA-MCC composite material are increased by 87.31% and 41.30%, respectively.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






