Covestro Launches Dureflex MA5000 TPU Film for High-Performance Medical Applications
Covestro, a world-leading supplier of high-tech polymer materials, has announced the official launch of Dureflex® MA5000. This innovative paper-carrier thermoplastic polyurethane (TPU) film is specifically designed for high-performance medical wearable applications, such as intravenous (IV) securement dressings and continuous glucose monitoring (CGM) patches.
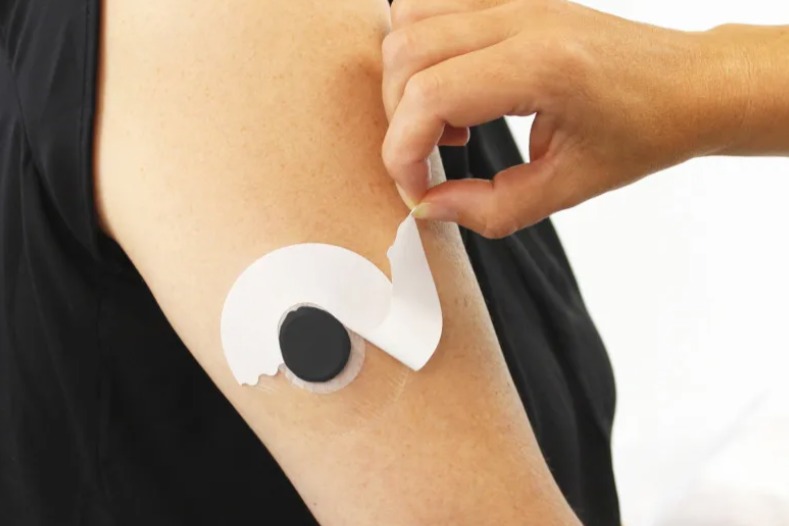
Soft films, intricate die-cutting, and high-temperature adhesive coating processes present significant challenges in the manufacturing of complex medical devices. Ensuring dimensional stability, cutting precision, and compatibility with high-temperature adhesive coating processes requires a stable and reliable carrier material. Dureflex MA5000 enables stable downstream processing, consistent product performance, and improved manufacturing efficiency.
Covestro stated that Dureflex MA5000 possesses three core advantages that can specifically address key industry pain points:
Designed for precision and stable processing: high carrier rigidity, stable and controllable release force, smooth transport, enabling precision processing, reliable demolding, and improved yield.
Supports high-temperature adhesive application: remains stable above 135°C, and the rigid carrier ensures uniform adhesive application, minimal deformation, and reliable release.
Provides structural support and simplifies device application: Maintains product form for precise application, supports staged application, and enables single-handed operation.
Dureflex MA5000 can be widely applied in various medical fields, including:
Intravenous indwelling fixation dressing
Continuous Glucose Monitoring (CGM) sensor patch
Scar management dressing
Medical wearables
Flexible Printed Electronics
Kidd Wang, Business Development Manager for Medical at Covestro, stated: "This product demonstrates Covestro's commitment to innovation in healthcare materials. By solving specific manufacturing challenges, we help our customers create more efficient and user-friendly medical devices, ultimately improving patient diagnosis and treatment outcomes."
【Copyright and Disclaimer】This article is the property of PlastMatch. For business cooperation, media interviews, article reprints, or suggestions, please call the PlastMatch customer service hotline at +86-18030158354 or via email at service@zhuansushijie.com. The information and data provided by PlastMatch are for reference only and do not constitute direct advice for client decision-making. Any decisions made by clients based on such information and data, and all resulting direct or indirect losses and legal consequences, shall be borne by the clients themselves and are unrelated to PlastMatch. Unauthorized reprinting is strictly prohibited.
Most Popular
-

Sony Joins 13 Chemical Material Firms to Build World's First Renewable Plastic Supply Chain in Electronics
-

Vioneo Abandons €1.5 Billion Antwerp Project, First Commercial Green Polyolefin Plant Relocates to China
-

Polyolefin Monthly Report: Stronger Cost Support, Focus on Downstream Follow-up Before the Holiday
-

BASF and Lanxess Announce Price Hikes! ExxonMobil Commissions Third Advanced Recycling Unit
-

Lutai's Major Equity Change! Uniqlo's Parent Company Joins Hands With Chenfeng to Enter the Market, Creating a "Community of Destiny" in the Textile Industry






