Wanhai Medical secures hundreds of millions of RMB in Series A financing! A review of common material categories for injection pens and upstream suppliers.
Recently, Jiangsu Wanhai Medical Devices Co., Ltd. announced the completion of a financing round worth hundreds of millions, exclusively invested by IDG Capital, with Yuan Yi Capital serving as the exclusive financial advisor. This financing will assist Wanhai Medical in further expanding its annual production capacity from the existing 90 million units to 310 million units, forming a total production capacity of 400 million units for an automated and intelligent injection pen production base, further consolidating its leadership position in the global injection pen market.

Wanhai Medical was established in 2016, headquartered in Xitai Lake, Changzhou, Jiangsu Province, with a branch in Shanghai. It is a national high-tech enterprise and a specialized and innovative enterprise. The company focuses on the research, development, production, and sales of various types of injection pen products. It currently has a factory area of 12,000 square meters and an annual sales revenue of hundreds of millions, serving over 180 customers globally, with business operations in more than 60 countries and regions. The company has more than 300 employees, including over 50 in the R&D team and 12 in the registration team. Wanhai holds over 50 product patents and has more than 30 product quality testing reports issued by third-party testing institutions. The core product patents have been ensured to be free of infringement risks for global sales through FTO searches conducted by a well-known patent agency. Wanhai has obtained CE certification from TÜV SÜD, ISO13485 certification, as well as domestic Class II medical device registration certificates, production licenses, and business filing certificates, and has completed national drug packaging material registration. The company is currently applying for FDA certification in the United States, which is expected to be completed by early 2026.
The demand for chronic disease management has given rise to the golden path of injection pens.
Chronic diseases such as diabetes and obesity have long been significant health threats to the public. At the recent press conference on people's livelihoods during the third session of the 14th National People's Congress, Lei Haichao, the director of the National Health Commission, emphasized the continuous promotion of the "Weight Management Year" initiative, urging the government, industry, institutions, and individuals to fulfill their responsibilities, and to continue effective knowledge dissemination on chronic disease prevention and weight management, while emphasizing the integration of prevention and treatment. GLP-1 receptor agonists have gained widespread application globally due to their remarkable efficacy in treating diabetes, obesity, and chronic diseases. In 2024, the global sales of Novo Nordisk's semaglutide alone are expected to approach $30 billion, and it is anticipated to become the world's top-selling drug by 2025.
The injection pen market has historically been dominated by foreign companies such as Switzerland's Ypsomed, Germany's Haselmeier, and Taiwan's SHL. Additionally, insulin giants like Novo Nordisk and Eli Lilly also have their own production capacity for injection pens. However, imported injection pens are often expensive, with the price of reusable injection pens ranging from tens to hundreds of yuan, and single-use injection pens priced between 12 to 20 yuan. The high cost of imported injection pens has become a significant factor limiting the accessibility of chronic disease medications. To effectively reduce the burden of medical expenses, China began including insulin in centralized procurement in 2021, which has provided a development opportunity for domestic injection pen companies. Furthermore, the ongoing deepening of economic and trade cooperation in the pharmaceutical field under the "Belt and Road" Initiative, such as the official launch of the China-ASEAN pharmaceutical regional procurement platform this January, has also opened up new opportunities for China's pharmaceutical procurement to "go global." More and more overseas countries are beginning to pay attention to the cost-effectiveness of medications and improving drug accessibility, which gives high-quality and cost-effective domestic companies a new chance to stand on the global stage.
Currently, Wanhai Medical has a stable production capacity of 90 million disposable and reusable injection pens per year, forming a vertically integrated industrial chain layout from mold manufacturing, injection processing, automated assembly to testing and inspection. The company has nearly 30 various mold production and processing equipment, mainly from Japan's Makino and Sodick. In terms of injection molding, the company owns nearly 70 high-end imported electric injection molding machines from Japan's Sumitomo Demag and Sodick, with raw material suppliers being global top brands. For assembly, the company has established multiple automated production lines and semi-automatic equipment, equipped with a cleanroom of class 100, and currently has a daily output capacity of 300,000 units. Additionally, the company is equipped with Hexagon coordinate measuring machines, Mitutoyo and Nikon optical measuring instruments, as well as high-precision analytical balances of class 10 and class 100, ensuring that the precision of the produced injection pens meets ISO 11608 requirements.
After nearly a decade of rapid growth, Wanhai Medical has achieved sales of hundreds of millions of RMB, with customers in over 60 countries and regions and more than 180 clients, including several top biopharmaceutical companies both domestically and internationally, such as Tonghua Dongbao and Biocon, India’s largest biopharmaceutical company, along with a number of other outstanding international partners. To better support domestic and international clients in scaling up major products such as insulin and GLP-1, Wanhai continuously reduces product costs through large-scale production and process optimization.
Related Reading
Composition of Injection Pen and Injection Molding Parts
The main components of the injection pen include the pen cap, pen body, pen core, pen core holder, spiral piston rod, knob, and injection push button.
Requirements for materials of injection pens
High rigidity and high dimensional stability
Excellent impact resistance, not易破碎when dropped.
Biocompatibility, high purity, stable product performance.
The sliding component materials need to have low friction properties to reduce injection force and prevent noise generation.
Gamma rays or ethylene oxide can be used for sterilization.
6. Recyclable materials
Common Material Categories and Upstream Suppliers for Injection Pens
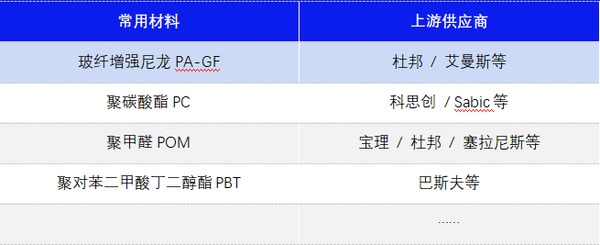
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






