UK equipment leader's first manufacturing base in China has been established and put into production.
01

As Keeler's first production base in China, the "China Factory" has undergone several key stages from project planning to final implementation, including technology transfer, factory construction, product registration, and production licensing.
The factory is equipped with a very strict quality management system to ensure that every piece of equipment produced meets the national medical device quality management system requirements. From raw material procurement to finished product delivery, every link undergoes rigorous inspection to fully guarantee the professionalism and high quality of the products.
Mr. Leon Forrest, Chief Operating Officer of Keeler, stated: "The completion of Keeler's factory in China and the implementation of localization projects demonstrate Keeler's commitment to deepening its presence in the Chinese market and long-term development. In the future, we will continue to innovate and integrate in all aspects of localization in production, service, education, and operations to better serve our customers in the China region."
02
Localized production will provide
China ophthalmology provides more treatment options.
Since producing the first binocular indirect ophthalmoscope, Keeler has accumulated many years of experience in the field of ophthalmic diagnostics. The localized production of the indirect ophthalmoscope will not only maintain the original high quality but also leverage local advantages to respond more quickly to market demands. As an essential device for ophthalmic examinations, Keeler's slit lamp microscope utilizes advanced optical technology to provide doctors with clear and accurate images of the eye, assisting in more precise diagnosis of eye diseases.
The binocular indirect ophthalmoscopy (BIO) examination is currently recommended as the "gold standard" for ROP screening, featuring economical and convenient characteristics. By using a scleral depressor, it can improve the diagnostic accuracy of peripheral lesions. It has good safety, a wide observation field, and strong stereoscopic vision, which can reduce the risk of missed and misdiagnoses. The Keeler indirect ophthalmoscope currently has five different models, represented by the Vantage Plus series.


Vantage Plus wired models: There are two power options, SmartPack and WallPack. The WallPack wall-mounted power supply is suitable for fixed installation in examination rooms and wards where the ophthalmoscope is regularly used. The SmartPack can be clipped to a belt or wristband for easy portability.
Slit lamp microscope
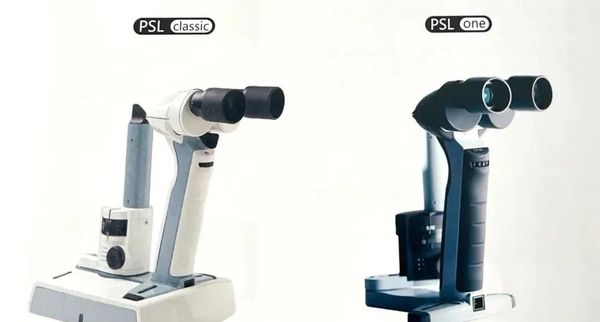
Desktop products such as KSL-H5-DR and KSL-H use advanced optical systems to provide exceptional clarity and precision, suitable for demanding daily ophthalmic environments.
Taking KSL-H5-DR as an example, the KSL-H5-DR uses Keeler optical components, providing the best optical performance in its class. This device uses selected LED lighting with a color temperature of 3800K, typically lasting 10,000 hours or longer, ensuring a stable and uninterrupted light source during the examination process.
03
The structure of the eye is intricate and complex, and high-end ophthalmic medical equipment is key to achieving precise diagnosis and treatment in ophthalmology. High-end ophthalmic medical devices can not only detect potential eye diseases early and assist doctors in managing them, but also help reduce the burden of eye diseases and the risk of blindness. Additionally, they can improve diagnostic accuracy, reduce surgical risks, enhance the safety and success rates of surgeries, and significantly improve treatment outcomes.
In recent years, with the rapid development of the Chinese economy and the gradual improvement of residents' health awareness, the ophthalmology medical market in China has shown a rapid growth trend. According to Sullivan analysis, the market size of ophthalmic medical devices (including contact lenses and care solutions) in China increased from 17.8 billion RMB in 2017 to 30.5 billion RMB in 2021, and is expected to grow to 55.6 billion RMB and 93.3 billion RMB by 2025 and 2030, respectively.
Under the attraction of this enormous market potential, many international ophthalmic giants such as Zeiss and Hoya are accelerating their localization efforts in China.
In October 2022, Zeiss launched the Suzhou R&D and manufacturing base project, which is set to officially commence operations in April 2024. The base covers an area of over 13,000 square meters, with a business scope that includes the R&D and manufacturing of industrial quality, research microscopes, surgical microscopes, and ophthalmic equipment. In addition, the third phase expansion project of the Zeiss Optical Industry 4.0 Health Optometry Industry Ecosystem was also announced in October 2024, with plans to build a modern factory in Huangpu District, Guangzhou, to further enhance local production capabilities.
In October 2024, the Hoya Group's Hoya Surgical Optics established its first artificial lens production base in Suzhou High-tech Zone. This base focuses on the development of artificial lenses and their implantation systems, further expanding Hoya Surgical Optics' global layout.
In fact, under the continuous promotion of national policies, the current development of the ophthalmic medical equipment industry in China has made significant progress. Domestic high-end ophthalmic medical devices are constantly emerging, and the process of localization is accelerating. In the field of high-end ophthalmic medical equipment, domestic alternatives are being achieved, with some areas realizing advantageous substitutions.
Industry analysis believes that the establishment of Keeler's factory in China will further intensify the competition in the Chinese ophthalmic medical device market. At the same time, this competition will become an important driving force for technological advancement and product innovation in the industry, bringing more new opportunities for the development of China's ophthalmic medical sector. As more international brands continue to implement localization strategies, what kind of new development stage will the Chinese ophthalmic medical device market usher in? Medical Device Home will continue to follow up on this.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






