The super-biological performance of immobilized enzymes is achieved through specific non-covalent interactions similar to those of molecular chaperones.

Abstract
Design of complex synthetic materials for enzyme immobilization can release the efficacy of biocatalysis in extreme environments. Inspired by biology, this article investigates the use of random copolymer brushes as dynamic immobilization carriers to impart super-biocatalytic properties to immobilized enzymes. This is achieved through the use of Bacillus subtilis lipaseAFixed on the brush with doped aromatic parts to demonstrate that the aromatic part can interact with lipase through various non-covalent interactions. The introduction of aromatic groups leads to an increase in the optimal temperature of the lipase.50℃, enzyme activity increased50times. single moleculeFRETThe study shows that these supports act as biomimetic chaperones by promoting enzyme folding and stabilizing the folded and catalytically active state of the enzyme. When aromatic residues are mutated, this effect is diminished, indicatingπstacking andπCationic interactions on the importance of stability. The research results emphasize unexplored enzymes-How support material interactions bring unknown opportunities for the use of enzymes in industrial biotransformation.
01, Introduction
Immobilization of enzymes on solid supports may provide higher stability, activity, and reusability for use in chemical and pharmaceutical manufacturing, biosensing, and bioremediation. Typical immobilization materials consist of highly porous carbohydrate-based materials, inorganic carriers, or composites, including agarose, inorganic nanoflowers, or metal-organic frameworks. However, due to the interaction between the enzyme and the material and/or the blocking of the enzyme's active site leads to denaturation, enzymes often become inactive when immobilized on these materials, which in turn can lead to a decrease in overall catalytic performance. Recent breakthroughs have shown that complex heterogeneous materials (such as lipid bilayers or copolymer brushes) have potential as customized carriers for enzyme immobilization. These synthetic materials possess extraordinary dynamics and chemical tunability, containing new chemical moieties that interact non-covalently with parts of the enzyme surface through transient self-assembly. Importantly, polymer brush supports are capable of introducing biomimetic non-covalent interactions, such as hydrogen bonding, hydrophobic self-assembly,πstacking andπcationic interactions. The latter's aromatic-related interactions exist in biomacromolecular components, including double strandsDNAextreme microorganisms' proteins and intracellular fluid-Liquid phase separation environment. This interaction is also believed to contribute to thermal stability, as they are insensitive to temperature changes and can achieve twice that of a salt bridge. Incorporating aromatic groups into the immobilization carrier can promote stability respectively.πaccumulation andπCationic interactions with aromatic or cationic surface-exposed enzyme residues.
Introducing bio-inspired stable interactions into dynamic and chemically heterogeneous polymer materials can achieve soluble enzymes-The complex stabilization mechanisms of polymer components or immobilized enzymes. For example, synthetic methods that mimic biologically relevant interactions have been explored using heteropolymer ensembles. Additionally, the polymer encapsulation of enzymes allows them to remain stable at high temperatures and at cosolvent interfaces. Although complex enzymes-Polymer interaction mechanisms may be difficult to unravel using traditional characterization methods, but a wide range of biophysical, biochemical, and computational approaches have been adopted. Additionally, single-moleculeFörsterresonance energy transfer (SM-FRETThe microscope provides unique mechanistic insights into enzymes fixed on a polymer carrier, while particularly demonstrating enzymes with appropriate regulation.-The dynamic carrier of polymer interaction equilibrium can stabilize the folding state and/or promote refolding to improve the performance of immobilized enzymes. The latter effect is similar to“class molecular chaperone”Mechanism, that is, the natural conformation and function of enzymes are rescued from the denatured state. Notably, this phenomenon has been reported for several natural and synthetic molecules as well as macromolecules, such as lipid bilayers or surface-grafted polymer brushes. These findings suggest that other variations in polymer structures may provide new opportunities for enhancing the performance of immobilized enzymes.
Previous studies have shown that the immobilization of Bacillus subtilis lipase A (LipA) on a zwitterionic sulfobetaine methacrylate (SBMA) polymer brush carrier promotes significant thermal stability of LipA. Unlike many other lipases, LipA lacks an amphiphilic lid covering the active site, thus its binding cleft is exposed to the solvent. It was found that the enhanced performance of LipA is due to the stabilization of hydrophilic interactions between the LipA surface and SBMA, with the LipA surface being overall highly hydrophilic compared to other lipases. Additionally, it was discovered that mixed polymer brushes with lower hydrophilicity, containing SBMA and poly(ethylene glycol) methyl ether methacrylate, can better stabilize more hydrophobic lipases. Here, the hypothesis that doping SBMA polymer brushes with varying amounts of aromatic ethylene glycol phenyl ether methacrylate (EGPMA) monomers can significantly enhance the activity and stability of immobilized LipA rich in aromatic residues at supra-physiological temperatures was investigated. Compared to previous efforts to stabilize enzymes using random copolymer brushes, the presence of aromatic EGPMA components may enable π-stacking and/or π-cation interactions. To further isolate the relevance of aromatic interactions, the effect of aromatic residue mutations in LipA on the stability of EGPMA incorporation into LipA was studied. Furthermore, SM-FRET was used to investigate the conformational dynamics of immobilized LipA, providing mechanistic insights into the complex unfolding and folding kinetics within the brush layer. Finally, the universality of this approach was demonstrated using another unrelated enzyme, carbonic anhydrase. Overall, the integrated observations at both macroscopic and molecular scales highlight the importance of properly tuned interactions between aromatic moieties in the immobilization carrier and surface residues of the enzyme for preserving and rescuing the folded and active state of immobilized enzymes.
02, Results and Discussion
1.SBMA/EGPMASynthesis and characterization of brushes
The purpose of this article is to study whether, compared to previous methods, it is possible to utilize a basedLipAstructural features of extensive non-covalent interactions to further enhance its stability. Interestingly,LipAhas an unusually high number of solvent-exposed aromatic residues, accounting for about of its surface7%。LipAThe aromatic patches found are less hydrophobic than the plaques formed by aliphatic residues, which are present in more hydrophobic lipases. In addition,LipAThe high surface hydrophilicity is also due to the presence of a large number of charged and hydrophilic residues (lysine, arginine, aspartic acid, glutamic acid). Based on these observations, the researchers hypothesizedLipAcan be achieved by entering inSBMABrushing in monomers with aromatic groups to further stabilize, these monomers may be associated with aromatics (πstacking) and cation (πcationic) residue-specific interactions. These aromatic groups may also promoteLipAthe other interactions, such as withLipAhydrophobic interactions of the solvent-exposed aliphatic portions around the active site, thereby potentially enhancing its overall stability and catalytic performance through these other mechanisms.
To verify the hypothesis, researchers prepared a material from the surface of silica nanospheres with a hydrodynamic diameter of 411 nm through atom transfer radical polymerization, using SBMA and 0-10% molar of aromatic
EGPMAcomposed surface-grafted random copolymer brush carrier (Figure1a). BesidesSBMAandEGPMA, the brush also includes5%Mole's glycidyl methacrylate (GMA),GMAcontains one epoxy group, which can covalently react with many sites on the enzyme surface, includingN-terminus, lysine, cysteine, tyrosine, and histidine. according topKaand solvent exposure analysis,LipAmost likely to be associated withGMAthe reaction sites includeN-terminal amine and histidine, includingC-end terminal6xhislabel. despite being rich inSBMAthe brush has protein resistance, but high reactivity at many sites makesLipAcan be covalently fixed. Assuming the reactivity of these methacrylate monomers characterized in previous work is the same, researchers estimate that for all brush supports, in the brush layerGMAthe average molecular volume is about the brush layer's2.5%, this indicatesLipAThe adhesion quantity between the brush layer is relatively independent of the brush composition. This was confirmed using diffuse reflectance Fourier transform infrared spectroscopy.EGPMAthe incorporation, the spectrum reveals withEGPMAincrease in content, aromaticC-HSystematic increase in stretching mode (figure1b). The characterization of polymer-modified particles by dynamic light scattering indicated that the solvated polymer corona thickness was73-143nm(figure1c). Finally, the researchers verified the wettability of all brushes grown on flat silicon dioxide wafers and found that the static contact angles of all surfaces were close to~11°, consistent with their highly hydrated state.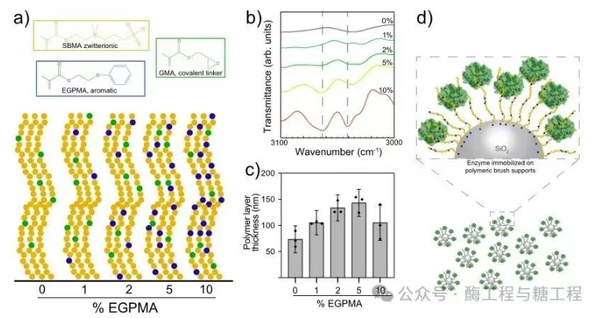
figure1. LipAin the mixSBMA/EGPMAOverview of immobilization on random copolymer brushes.apossessesSBMA、EGPMAandGMASchematic diagram of the brush surface of chemical structure.bgrown on silica nanoparticles0-10%EGPMApolymer brushFTIR-DRIFTSspectrum. Dashed line represents aromaticC-Hstretching mode characteristic peakcThe thickness of the hydrated polymer layer measured by dynamic light scattering. For each composition, subtract the core from the radius of the polymer-grafted particles.SiO2The radius of the particles. Error bars represent three technical replicates.n=3the standard error of the mean, taking into account the error propagation related to the subtraction of core silica particles.dbrush layerLipAandGMAcombined support diagram obtained
2. SBMA/EGPMAmixed carrier endowmentLipAsuper bioactivity and stability
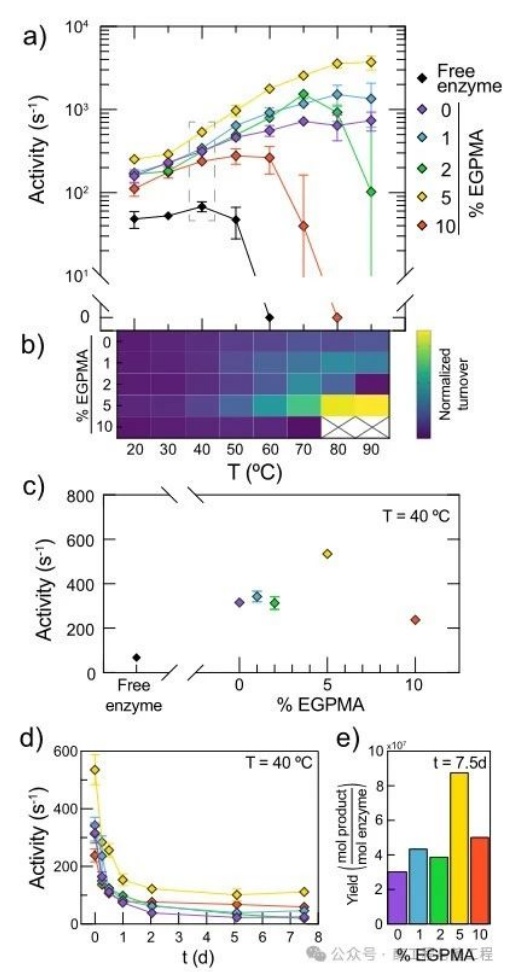
to determine the immobilizationLipAthe activity and temperature in the coating layerEGPMAthe relationship of content, willLipAcovalently fixed on0-10%ofEGPMAon the carrier, load per gram of carrier0.16-0.38mgenzyme. by measuring the initial hydrolysis rate of butyric resorcinol and20-90℃the temperature relationship, analyzed the free and immobilizedLipAthe stability. Researchers observed that, for all polymer brush compositions,LipAthe fixation leads toLipAActivated and stabilized at high temperatures. Interestingly, immobilizationLipAthe activity at high temperatures strongly depends on the coating layerEGPMAcomponents. This can be in the figure2aseen in the figure2ashowed free and immobilizedLipAthe temperature-dependent activity curve. In addition, onLipAthe stabilizing effect along withEGPMAcontent as high as5%and increase, then for those containing10%EGPMAthe carrier and decrease. Specifically, compared to freeLipAthe optimal measurement activity (Vopt) compared to,LipAin5%EGPMAImmobilization on the carrier leads to an enhancement of catalysis up to50times, while the optimal activity temperature (Topt) from40℃rise to90℃. In addition, although freeLipAin40℃The above shows a significant loss of catalytic activity, but fixed in5%EGPMAon the carrierLipAexhibits increasingly higher activity throughout the entire measured temperature range. Notably,10%ofEGPMAthe stability of the carrier is lower than that of the pureSBMAcarrier (excluding)EGPMA). This non-monotonic trend indicates that, withEGPMAthe subtle stabilization mechanism related to the best score. For ease of visualization, the diagram2afixed in the middleLipAthe different performances (i.e., turnover rates) with changes in temperature are represented as a heatmap (figure2b). This highlights when fixed in5%EGPMAon the carrier,LipATurnover rate increases monotonically with temperature, and0%、1%and2%EGPMAthe activity of the carrier is significantly reduced,10%EGPMAcarrier in70℃The above activity is lost. To further illustrateLipAnon-monotonic relationship between activity and brush components, figure2cshowed the enzyme in40°Cthe turnover rate andEGPMAthe relationship of content
To better understand the potential phenomena associated with the enhancement of LipA catalysis, researchers analyzed the kinetic parameters of immobilized LipA as a function of temperature. This analysis showed that the catalytic differences between carrier compositions at high temperatures could be attributed to differences in kcat rather than Km. However, the increase in kcat was not sufficient to explain the entire reason for the enhanced catalysis of LipA. This suggests an interaction between the increase in kcat and other synergistic effects, such as increased active site preservation, with temperature. The Km analysis indicated that, at a constant temperature, the differences in substrate binding as a function of EGPMA content were negligible. Furthermore, although the Km values for all brushes were higher at high temperatures, indicating weaker substrate binding, this might be due to reduced absorption of the substrate into the brush layer as determined by measuring substrate distribution. These findings collectively suggest that the apparent enhancement in catalysis can indeed be explained by the thermal stabilization of LipA on the brush surface. Previous studies have shown that complex heterogeneous carriers can extend the lifespan of immobilized enzymes. To investigate the ability of EGPMA-containing carriers to maintain enzyme activity, researchers measured the time-dependent catalytic activity of immobilized LipA by incubation at 40°C (Figure 2d). Within one day, the relative activity decrease for all carrier compositions was similar, although the 5% EGPMA carrier promoted higher initial LipA activity. However, certain carrier compositions, specifically 5% EGPMA, were able to maintain the activity of immobilized LipA for a longer period. To visualize the cumulative activity over time, researchers estimated the reaction yield of product formation by integrating the data in Figure 2d, which represents the area under the activity retention curve. This analysis showed that the 5% EGPMA carrier provided at least twice the specific product yield (Figure 2e), highlighting the practical relevance of fine-tuning the EGPMA content in immobilization carriers for stabilizing LipA at high temperatures.
Based on the above stability results, researchers attempted to elucidate the mechanism of aromatic interactions onLipAThe impact of stability. If this interaction contributes toLipAthe stability, then if the aromatic residues in the enzyme are removed,EGPMAThe stabilizing effect should decrease. By designing three differentLipAvariant to test this hypothesis, where the relative amount of surface-exposed and buried aromatic residues is mutated to alanine (exposed) or methionine/Valine (buried). The mutant is namedLipAΔSA-50%, its solvent-accessible aromatic surface residues undergo mutation, reducing the aromatic surface area50%,LipAΔSA-100%, all of its surface aromatic residues have been removed, andLipAΔburied-100%, with all its buried aromatic residues removed. These mutants allowed researchers to determine the removal of solvent-exposed aromatic residues available in the folded state (LipAΔSA-50%andLipAΔSA-100%) whether it will lead to stableπaccumulation andEGPMAofπ-Cationic interactions decrease. In addition, the removal of buried aromatic residues available in the unfolded state (LipAΔburied-100%) enable researchers to test in the unfolded state withEGPMAthe reduction of aromatic interactions also leads to a decrease in activity and/or an overall decrease in stability, which may enhance refolding.
The mutants were immobilized on particle carriers containing EGPMA, with each gram of carrier loaded with 0.14-2.28mg of enzyme, and their catalytic activity was measured. The heat map of activity shows that LipA mutants with reduced aromatic content (exposed and buried) are stabilized on polymer carriers with lower aromatic components than the wild-type LipA (LipAWT), even though these mutants have lower stability after optimal immobilization. Specifically, compared to the optimal 5% EGPMA support for LipAWT, all three mutants showed the best stability on supports with lower EGPMA content (1% or 2%) (Figure 3). Additionally, compared to immobilized LipAWT, all three immobilized mutants had reduced thermal stability, losing activity above 70°C, although some soluble mutants were more active and/or stable than soluble LipAWT. These findings suggest that the aromatic residues in LipAWT play a significant role in promoting stable interactions with EGPMA-containing carriers, supporting the hypothesis that π-stacking and π-cation interactions are involved in the stabilization of immobilized LipAWT. Other potential mechanisms may also be relevant, including the formation of hydrophobic niches within the brush layer, which could promote hydrophobic interactions. Changes in aromatic content may also alter the rigidity of the polymer chains, potentially affecting the self-assembly of the brush layer around the folded and unfolded states of the enzyme. Furthermore, to determine whether mutations in LipA affect substrate binding, the researchers measured the Km of the soluble form of each variant and found that the Km values for LipAWT, LipAΔSA-50%, and LipAΔburied-100% were similar, while the Km for LipAΔSA-100% was higher than the other variants. The latter is not surprising, as one of the mutations in LipAΔSA100%, Y161A, is near the active site and may affect substrate binding. Even with this difference, the variation in Km is not sufficient to explain the observed differences in activity.
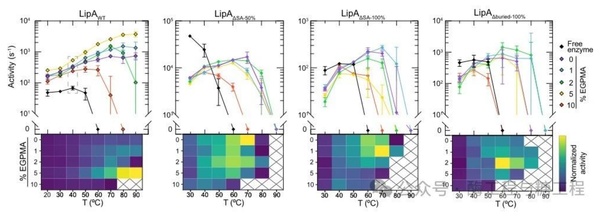
figure3. surface exposure and buried aromatic residue mutationsSBMA/EGPMAmixed brushingLipAthe impact of activity. Activity asEGPMAconcentration function represented by various mutants and wild typeLipAthe figure and the corresponding heatmap. For each heatmap, the color scale is based on the lowest and highest activities of the wild-type and mutant immobilized enzymes. The crosshatched rectangles correspond to conditions where there is no measurable activity for the immobilized enzyme and the wild-type enzyme. Note,LipAWTthe diagram and heatmap with the diagram2aRepetition. The error bars in all figures represent the standard error of the mean of three technical replicates for each measurement.n=3)。
4. single moleculeFRETdetermination of the conformational state of immobilized enzymes
SM-FRET microscopy for studying brush chemistry on immobilizationLipAThe mechanism basis of stability impact. Compared with the integrated and time-averaged measures of traditional protein stability,SM-FRETprovides quantitative information on the dynamics of the conformational states of individually immobilized enzymes. In this workSM-FRETthe experiment will highly reuse single molecule localization andFRETcombined withnmlevel resolution measurement of conformational changes in a single enzyme molecule.
forSM-RETexperiment, used the previously reportedLipAvariant, this variant has cysteine and non-canonical amino acid p-azido-phenylalanine exposed to the solvent. This allows for the use of orthogonal click chemistry toFRETmatchedAlexa Fluor 555andAtto 647 Nspecific markerLipAsite (LipA-FRET, diagram4a), this has been obtainedSDS-PAGEthe confirmation. The position of the fluorophore keeps it away from the active site andLipAfolded state andR0=4.59 nmofFörsterradius is very close (approximately2.4 nm), leading to highFRETThe donor and acceptor are also far apart in the primary sequence, leading to a large separation in the unfolded state. This separation is modeled by self-avoiding random walks.LipAthe random coil extension state to estimate, resulting in an average inter-fluorophore distance of about9.8 nm. Therefore,LipA FRETAfter unfolding, it is expectedFRETwill significantly decrease. By making the freeLipA FRETconducting chemical denaturation, this confirmed it, which led toFRETEfficiency systematically decreases with changes in the concentration of the denaturant, indicatingLipAthe tertiary structure is destroyed.
LipA-FRETfixed on the imaging silicon wafer by0%、5%and10%EGPMAon the brush composed ofSM-FRETexperiment in20°Cand45°CThe process was carried out under different conditions, providing information on the stabilization mechanisms under both thermal stability and thermal denaturation. At each temperature, a large number of trajectories were collected to allow for statistically rigorous measurements of time-averaged quantities (such as average folding scores) and dynamic parameters (such as apparent unfolding and folding rates). Donor-Receptor histogram heatmap is generated based on all observations of each brush component, used to identify fixation in each experimental implementation.LipAthe folding and unfolding status group (figure4b). In these two-dimensional histograms, the population with low acceptor intensity and high donor intensity corresponds to the apparent unfolded state, while the population with high acceptor intensity and low donor intensity corresponds to the apparent folded state. For each brush component, the populations are defined using a minimum integrated intensity threshold line as shown in the figure.4bas shown. The average time-fold score for the composition of each type of brush (ϕFis calculated by dividing the number of folded state observations by the total number of observations.
The analysis of the folded part unexpectedly showed,5%ofEGPMAcarrier can maintain folding under thermal denaturation conditionsLipAconformation. specifically, in20°Cunder non-denaturing conditions, immobilizationLipAin0%EGPMAcarrier (i.e.SBMAhomopolymer exhibits the highest folding score,F=0.89±0.04, andLipAin5%and10%EGPMAonF=0.72±0.01and0.66±0.04Lower. It is worth noting that, in45°Cbelow5%ofEGPMAcarrier maintained with20°Cunder the same folding level (ϕF=0.73±0.02), and fixed in0%and10%EGPMAon the carrierLipA-FRETshows reduced stability (respectively\981]F=0.56±0.04and0.60±0.02)。
These data indicate that all three carrier compositions exhibit significant conformational stability under thermal denaturation conditions. Notably,45°Chigher than solubilityLipA67ofTm, the researchers' activity measurements found freeLipAofToptfor~40°C; therefore, solubleLipAin45°Cmost of the following is expanded. Therefore, in45°Cat, fixed on all three carriersLipAofϕFthe non-zero value can be attributed to good brushing-enzyme interaction, and with the figure2afixed in itLipAthe activity retention remains consistent.
Interestingly, visual inspection of the FRET heatmaps revealed that the LipA-FRET unfolded state population immobilized on 5% EGPMA support was more compact (i.e., less diffusive) than the unfolded state populations immobilized on 0% and 10% EGPMA supports. This suggests that, compared to other surfaces, the unfolded LipA-FRET immobilized on 5% EGPMA carrier is more conformationally constrained. This difference can also be observed in the distribution of donor intensity for the unfolded states on each surface, indicating that 5%
ofEGPMASupport does indeed promote a more constrained expansion state.
figure4. LipA-FRETschematic and fixationLipA-FRETofFRETheatmapamarkedLipA-FRETschematic, with average distance between fluorophores (Ravg)。b FRETHeat map corresponds to each polymer carrier composition (rows) and each temperature (columns). The dashed white line indicates the threshold used to distinguish between folded and unfolded observations. Annotated numbers represent the average folding score under each condition.ϕFthe relevant values. The numbers in parentheses indicate five trials of dashed line measurements using different starting points before the unsupervised minimization method (n=5the standard deviation of the folding score.
5.The composition of the regulating brush reduces unfolding and accelerates refolding kinetics
To further understand under thermal denaturation conditions, fixed in5%EGPMAon the carrierLipAimproveϕFthe mechanism, fromSM-FRETTrajectory dynamic information was extracted. Specifically, the researchers identified immobilizationLipA-FRETthe transition probability between the folded and unfolded states, and use them with three statesMarkovchain model calculated the effective kinetic rate constants for unfolding and folding under each experimental conditionkunfoldandkfold) (figure5a). In20°Cat the time, along withEGPMAthe increase in content,ϕFThe significant reduction is associated with a systematic decrease in the reconstitution rate. Notably, in20°Cbelow0%EGPMAbrush'skfoldmaximum (6.54±2.10 s-1), and asEGPMAcontent increases to5%(3.92±2.34 s-1) and10%(1.53±0.22 s-1) and systematically reduced. At the same time,0%(0.156±0.047 s-1) and5%(0.120±0.061 s-1)EGPMAbrushedkunfoldsimilar10%EGPMAbrush higher (0.310±0.041 s-1)。
in45°CThe following similar analysis reveals a subtle synergistic effect, which promotes refolding by inhibiting unfolding and facilitating renaturation.5%EGPMAbrush on fixationLipA FRETthe higherϕF. First,5%ofEGPMAbrush ratio0%(2.13±0.29 s−1) and10%(1.11±0.17 s−2) ofEGPMAsupport has been enhancedkfold(2.40±0.55 s−3). Meanwhile, and0%(0.922±0.127 s-1) and10%(0.430±0.061 s-1)EGPMAbrushing ratio,5%EGPMABrush suppression of immobilizationLipA-FRETthe unfolding dynamics, itskunfoldlower (0.298±0.063 s-1). It is important that,5%ofEGPMAbrush up“class molecular chaperone”the interaction between faster folding and slower unfoldingϕFthe significant increase is related, thereby enhancing the catalytic performance. For the first approximation,LipAin5%EGPMAThe behavior on the carrier can be explained by a stable folded state and an unstable unfolded state.
When the protein is in one state or another, by tracking the root mean square fluctuations of the distance between fluorophores (RMSF) to measure the fluctuations in folded and unfolded states (Figure5b). for each stateRMSFvalue analysis revealsEGPMAthe subtle dynamic trend of content, in20°Cand45°Cdetermine the nature of similarity. Specifically, fixationLipAcollapsed stateRMSFasEGPMAthe content decreases systematically with the increase, indicating the protein-polymer-The increase in aromatic interactions may restrict the movement of proteins. This is related to5%and10%EGPMAbrush relative to0%EGPMAofkunfoldreduction is consistent. The fluctuation of the expanded state withEGPMAthe increase in concentration rather than monotonic change,5%EGPMAbrush on expanded stateRMSFreduce5%EGPMABrushing significantly promotes a unique unfolding state, which is less dynamic and more conformationally constrained; compared to other brush combinations that promote less restricted unfolding states, this unfolding state refolds faster.
The analysis of transition rate constants and fluctuations indicates that the aromaticity of EGPMA plays a crucial role in the folding and unfolding behavior of LipA. Specifically, it is hypothesized that monomer residue π-stacking and π-cation interactions may occur between the phenyl moiety of EGPMA and the numerous solvent-exposed tyrosines, phenylalanines, and tryptophans (π-stacking) of LipA; or with lysines and arginines (π-cation), impacting the kinetics and stability of catalytically active LipA. For instance, the folded state of LipA can be promoted by stabilizing aromatic interactions, effectively lowering the free energy of the folded state through decreased kunfold. Conversely, upon unfolding, previously buried phenylalanines or tyrosines can form new π-stacking interactions with EGPMA, as well as π-cation interactions with the quaternary ammonium of SBMA. Since the timescale of polymer side-chain fluctuations is several orders of magnitude faster than enzyme unfolding, the brush can probe newly exposed residues and self-assemble around previously buried aromatics. Researchers speculate that these interactions may facilitate refolding by effectively trapping the enzyme in a partially unfolded state during the unfolding process, leading to the locally and dynamically restricted unfolded states observed with 5% EGPMA support. This, in turn, may decrease the probability of LipA entering a slow or kinetically trapped unfolded state. Therefore, by inhibiting the unfolding process, optimally regulated 5% EGPMA carriers can help LipA return to its folded, catalytically active conformation, consistent with the accelerated kfold relative to other polymer compositions.
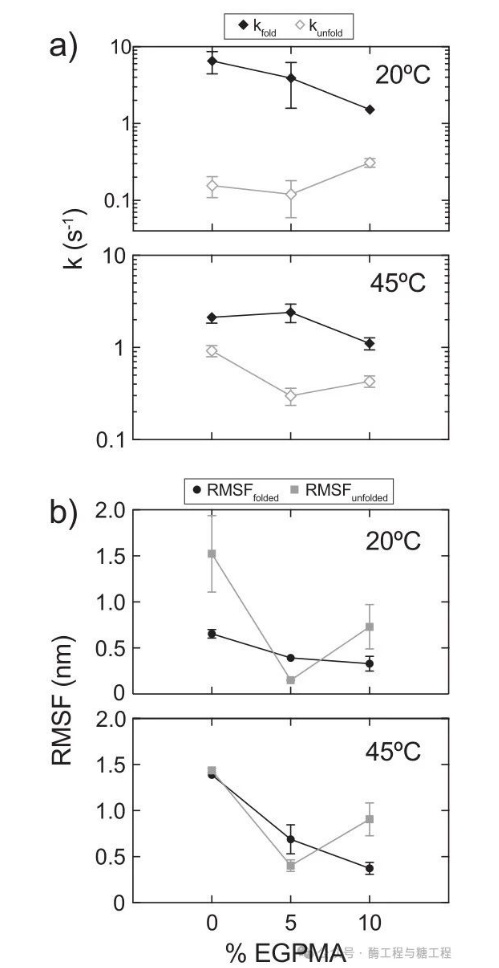
figure5. fixed inSBMA/EGPMAbrushed onLipA-FRETthe dynamic analysis.aThe effective transition rate constants for folding and unfolding under each experimental condition. Error bars represent the standard error of the rate constants, byKienleas stated by othersCramèr-Raothe square root of the lower limitbThe fluctuations of folded and unfolded states are respectively represented by the root mean square fluctuations of individual trajectories when the protein is maintained in the folded or unfolded state.RMSF) measurement. Error bars were estimated for each conditionRMSFten iterations of measurementn=10the standard error of the mean.
6. containsEGPMAstability of carbonic anhydrase on the brush
to determine that it containsEGPMAthe brush can be used to stabilize in structure and function withLipAdifferent enzymes, researchers chose a human carbonic anhydraseII(hCAII) of the heat-resistant mutant. Carbonic anhydrase has potential applications in carbon capture because it can sequester carbon dioxide as carbonate through hydration.hCAIIfixed in containingEGPMAon the brush, per gram of carrier load0.81-8.60 mgenzyme, and use4-Methylumbelliferyl acetate as a fluorescent substrate, measuring the change in enzyme activity of free and immobilized enzymes with temperature (figure6). Although free enzymes are more active than immobilized enzymes at low temperatures, the results show that all supports improvedhCAIIthe thermal stability, which indicateshCAIIThere is a trade-off between activity and stability. In addition, thermal stability increases withEGPMAthe systematic increase with the increase in content,10%ofEGPMAThe carrier is more stable than other compositions, achieving higher levels than free enzymes9times the optimal activity. Importantly, all containingEGPMAthe carrier's catalytic performance at high temperatures exceeded that of the free enzyme, while only those not containingEGPMAthe support body failed to exceed the free in activityhCAIIThese observations demonstrate thatEGPMAsupport object promotionhCAIIstability and the correlation of increasing activity
checkhCAIIthe structural characteristics, finding that it contains many that may be associated withEGPMAthe aromatic part has favorable interactions with residues. For example,hCAIIcontains23an exposed lysine and7an arginine residue, all of which can participateπ-cationic interactions. In addition, withLipAcompared toLipAthe solvent exposure of is greater than that of the buried aromatics,hCAIIcontains11individual solvent exposure and16a buried aromatic residue, these residues may be associated withEGPMApartialπstacking related. based onhCAIIthe results of the study on activity and structural characteristics, it can be concluded thathCAIIfixed in containingEGPMAon the carrier has greatly improvedhCAIIthe thermal stability and activity, which may be due tohCAIIand between the layers multipleπcation andπStacking interactions. Overall, these results summarize the observations of this article, indicatingEGPMAThe carrier has significant stability for enzymes rich in aromatic and cationic residues.
Polymer brush carriers containing zwitterionic/aromatic (SBMA/EGPMA) random copolymers significantly improved the catalytic performance and thermal stability of immobilized LipA (an enzyme with high aromatic content). Specifically, compared to free enzymes, the optimized 5% EGPMA support promoted a 50°C increase in Topt and a 50-fold increase in Vopt. Mutants of LipA with reduced exposed or buried aromatic content were stabilized to a lesser extent, indicating that stabilization may involve π-stacking and/or π-cation interactions between aromatic groups in the brush. SM-RET analysis showed that 5% EGPMA brushes more effectively maintained the folded state of immobilized LipA, preventing thermal denaturation, compared to other SBMA/EGPMA carriers. The stabilization of the folded state is consistent with the reduced unfolding kinetics of LipA immobilized on 5% EGPMA brushes. Enhanced refolding kinetics of transiently unfolded enzymes and SM-FRET fluctuation analysis indicated that immobilized LipA on 5% EGPMA brushes exhibited a highly restricted unfolded state, which was prone to refolding, possibly explaining the enhanced refolding kinetics.
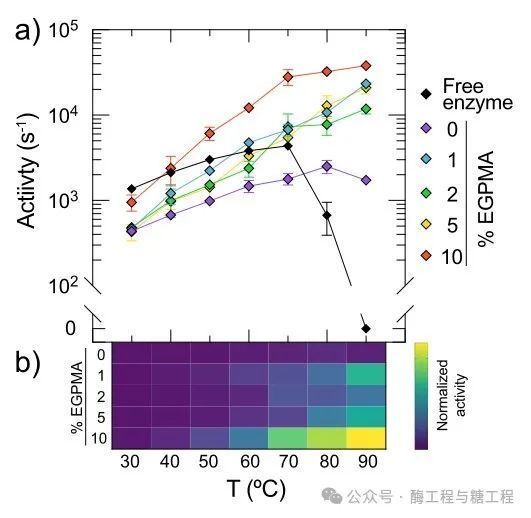
figure6. mixedSBMA/EGPMAbrush uphCAIIthe stability.SBMA/EGPMAcarrier-fixed and freehCAIIthe temperature-dependent activity. Error bars represent the standard error of the mean for three technical replicates of each measurement.n=3)。bheatmap corresponds to the panelathe temperature-dependent activity data, scaled by the interval between the lowest and highest turnover rates of the immobilized enzyme.
03, Conclusion and Prospects
These findings highlight the potential of complex materials to confer super-biological properties on enzymes, while also opening up opportunities for exploring new heterogeneous polymers. Future work will explore further stabilizing enzymes in increasingly complex carriers, including those that also contain charged, hydrophobic, and hydrogen bond donor groups./receptor or strong dipole multicomponent mixtures. Ultimately, these new materials will provide unprecedented opportunities, allowing the use of enzymes in harsh environments, thereby catalyzing extremely difficult and interesting synthetic biotransformations. By promoting enzyme stability in such environments, the researchers' findings may have a significant impact on the optimization of biological processes and the engineering of biological system reactions.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






