Taiyuan Institute of Technology: Preparation and Properties of Bio-based Semi-aromatic PA5T-co-56
Abstract: The melting temperature of the semi-aromatic poly(pentamethylene terephthalamide) (PA5T) homopolymer is close to its thermal decomposition temperature, making it prone to decomposition during melt processing. To address this issue, a copolymer (PA5T-co-56) of PA5T and poly(pentamethylene adipamide) (PA56) was synthesized using pentamethylenediamine, adipic acid, and terephthalic acid through a salt formation and polymerization process. The structure of PA5T-co-56 was analyzed by Fourier transform infrared spectroscopy and nuclear magnetic resonance spectroscopy. Based on this, the thermal properties and crystallization performance of PA5T-co-56 were tested using differential scanning calorimetry, thermogravimetric analysis, and X-ray diffraction, and its solvent resistance was characterized. The results showed that with the increase in the content of the PA56 segment in the copolymer structure, the crystallization temperature and melting temperature of PA5T-co-56 gradually decreased. When the molar ratio of the PA56 segment was 40%, 50%, 60%, and 70%, the melting temperature of the copolymer decreased by 56.42, 74.70, 83.03 °C, and 91.66 °C, respectively, compared to that of PA5T. This is mainly due to the introduction of the PA56 segment, which reduces the proportion of rigid benzene rings in the polymer molecular chain and increases the flexibility of the molecular chain. The introduction of the PA56 segment did not significantly reduce the thermal stability of the polymer, nor did it change the main crystal form of the polymer, which remained primarily γ-form.
Polyamide (PA) resin can be formed by the self-polymerization of lactam molecules or by the condensation polymerization of diamines and dicarboxylic acids. According to their main chain structure, they can be classified into aliphatic PA, semi-aromatic PA, and fully aromatic PA [1-3]. Among them, the most widely used aliphatic PAs, PA6 and PA66, have excellent physical and mechanical properties, wear resistance, self-lubrication, and noise absorption performance, and have been extensively applied in fields such as automobiles, machinery, and electrical appliances [2-6]. With the development of surface mount technology (heat resistance temperature ≥ 270 ℃) and the higher requirements for heat resistance and weather resistance of materials in fields like optics, aerospace, and military, the performance of aliphatic PAs such as PA6 and PA66 has become difficult to meet the usage demands [7-8]. Fully aromatic PAs, with a high density of rigid benzene rings and excellent heat resistance, have a thermal decomposition starting temperature lower than the melting temperature, making it difficult to process through melting, which limits their further application. Semi-aromatic PAs, containing both flexible aliphatic segments and rigid benzene ring segments, combine the good processability of aliphatic PAs with the outstanding heat resistance of fully aromatic PAs [9].
Currently, the semi-aromatic PAs that have been industrialized mainly include copolymers of PA6T, PA9T, and PA10T[10-12]. Compared to PA10T and PA9T, PA6T has better heat resistance, lower cost, and greater market competitiveness. However, the development of petroleum-based product PA6T is limited by China's energy situation characterized by abundant coal, scarce oil, and limited gas, as well as the environmental pressure of carbon peak and carbon neutrality[13]. The structure of pentamethylene diamine is similar to that of hexamethylene diamine, and BioAmber Inc. has already industrialized bio-based pentamethylene diamine[14-16]. Based on the above background, using bio-based pentamethylene diamine and terephthalic acid as the main raw materials, bio-based poly(pentamethylene terephthalamide) (PA5T) was prepared. On this basis, aliphatic poly(pentamethylene adipamide) segments (PA56) were introduced into the main chain of PA5T to prepare a copolymer of poly(pentamethylene terephthalamide) and poly(pentamethylene adipamide) (PA5T-co-56) with a wider processing window. The structures of PA5T and PA5T-co-56 were confirmed, and their thermal properties, crystallization performance, and solvent resistance were tested and characterized.
1 Experimental Section
1.1 Main Raw Materials
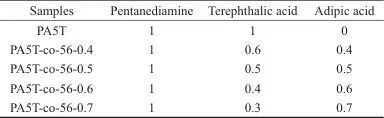
Table 1 Molar ratio of raw materials for PA5T and PA5T-co-56
1.4 Preparation of PA5T and PA5T-co-56
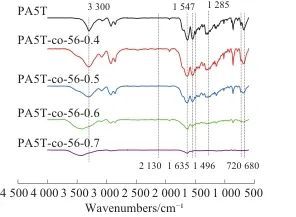
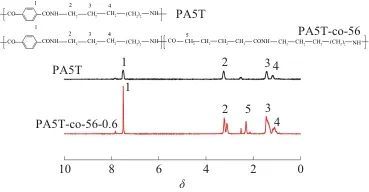
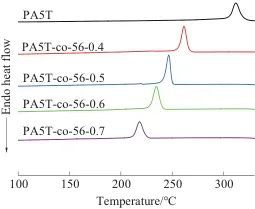
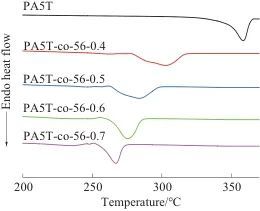
Table 2 DSC and TG test data of PA5T and PA5T-co-56 ℃
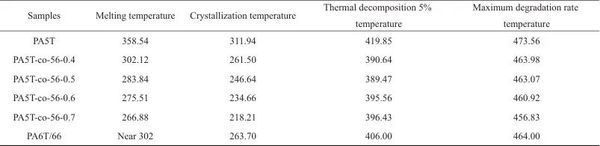
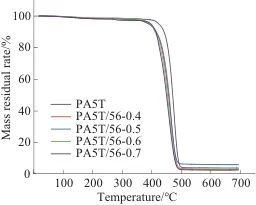
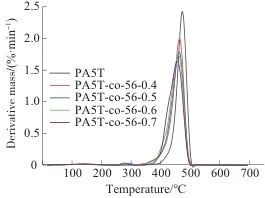
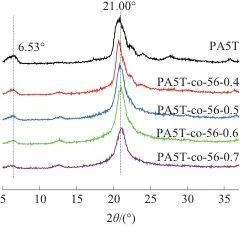
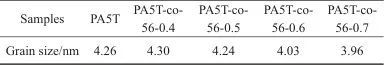
Table 3 Grain size of PA5T and PA5T-co-56
2.5 Solvent Resistance Analysis
Table 4 Solvent resistance test results of PA5T and PA5T-co-56
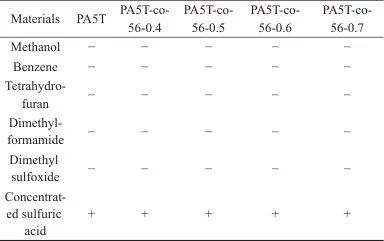
Notes:+ means dissolution;- means non-dissolution.
3 Conclusion
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






