Shanghai Institute of Organic Chemistry, degradable carbon backbone acrylic polymer! Latest in Nature Chemistry
Researcher Hong Miao from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Laurent Maron from the University of Toulouse III collaborated to publish a groundbreaking study in Nature Chemistry, successfully developing a novel degradable carbon backbone acrylic polymer based on coumarin (CM). This material not only possesses mechanical and optical properties comparable to those of traditional polymethyl methacrylate (PMMA), but under the action of strong alkali, the alternating copolymer can utilize aromatization as a thermodynamic driving force, effectively breaking the main chain C-C bonds to completely degrade at room temperature, producing pure, pharmacologically valuable molecules, thus obtaining a durable, robust, and fully degradable carbon backbone acrylic polymer.

【Research Background: The Challenge of Carbon Skeleton Plastic Degradation】
Data shows
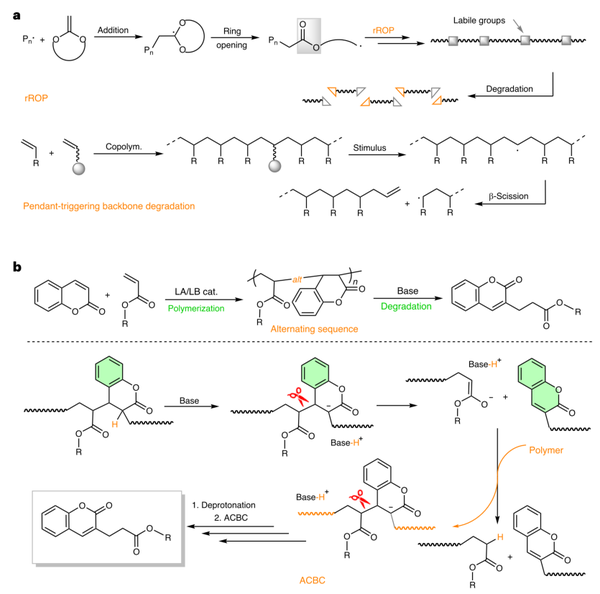
Figure 1. Different strategies for producing degradable vinyl polymers.
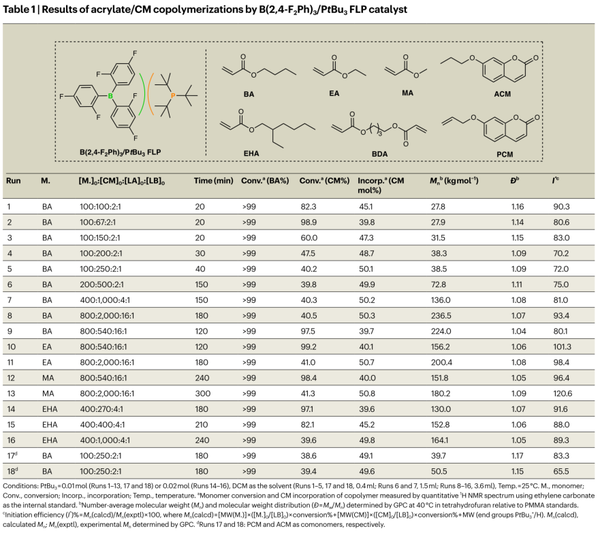
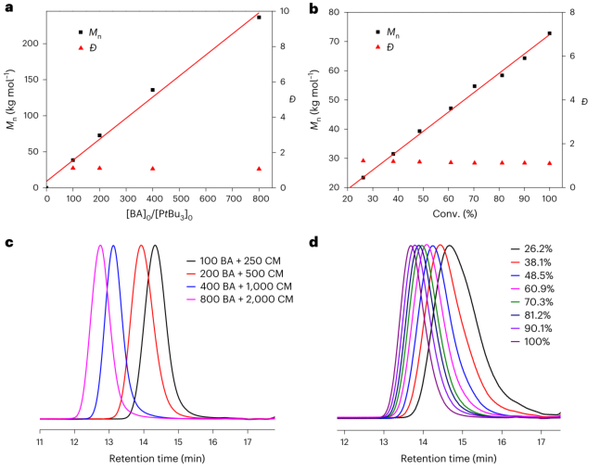
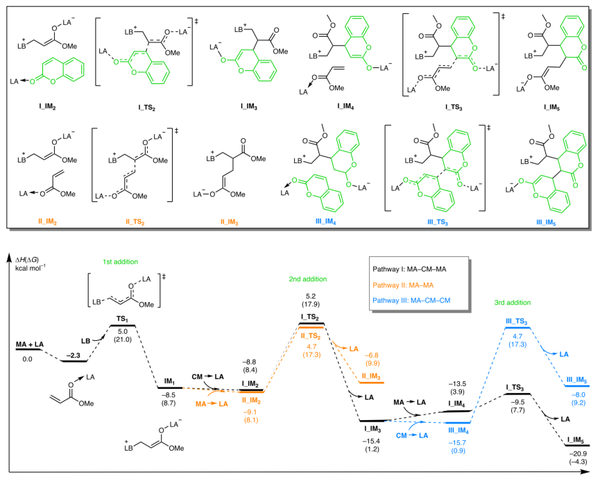
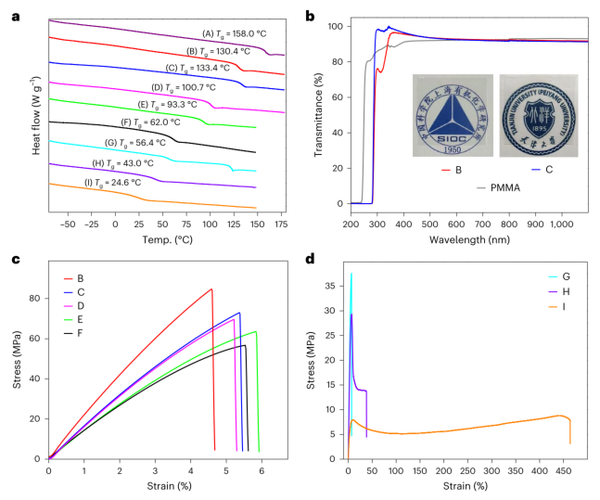
Figure 4. Physical properties of acrylate/CM copolymer.
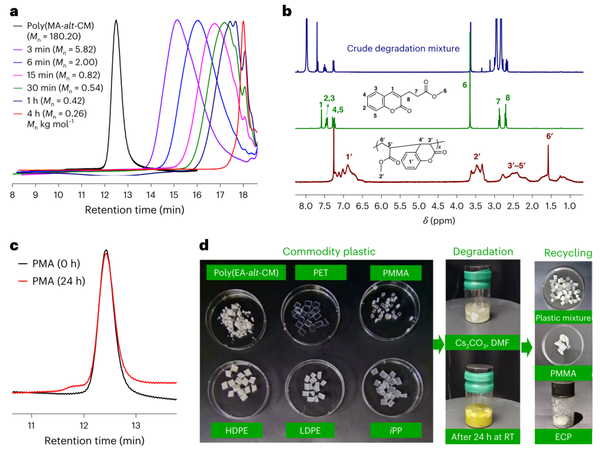
Figure 5. Degradation behavior of acrylate/CM copolymer.
[Insights from the Results]
This study achieved the efficient, active, and alternating copolymerization of acrylates with CM for the first time by developing a novel B(2,4-F2Ph)3/PtBu3FLP synergistic catalyst. Incorporating simple, commercially available, and biorenewable CM into the main chain has two aspects:
Providing new carbon backbone acrylic polymers as robust, transparent thermoplastics, whose key thermal, optical, and mechanical properties are comparable to or better than PMMA, thanks to the rigid cyclic structure of CM units;
(2) More importantly, the effective cleavage of main-chain C-C bonds is achieved by utilizing aromatization as a thermodynamic driving force (ACBC). Under the action of strong bases, the alternating copolymers can be completely degraded into pure and pharmacologically valuable molecules at room temperature.
The unprecedented chemical reaction introduced in this article provides new durable, robust, and fully degradable acrylic polymers that will find practical applications in a variety of fields, ranging from sustainable plastics to functional polymers such as nanolithography.
Finally, if advanced catalysts that can be incorporated into CM can be designed, then the ACBC strategy could also make energy-saving degradation/depolymerization of other carbon backbone polymers (such as polystyrene, polyvinyl chloride, polybutadiene, and even polyethylene) feasible.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






