New Breakthrough! Yuan Tian Biotech Produces the World's First Bio-Enzymatic Regenerated PET Bottle from Waste Textiles
Currently, the 300-ton/year regenerated PTA production line of SourceSky Biotechnology is already in stable mass production. In terms of products, it has completed a series of validations from rPTA to rbPET to spinning to weaving to finished garments and shoes, successfully exploring the closed-loop application of recycled polyester in the textile industry. In packaging materials, it has produced the world's first bio-enzymatic regenerated PET bottle using waste fiber as raw material, completed partial film production validation, and is continuously expanding downstream customers, enabling bio-enzymatic regeneration technology to be applied in more industries.
From March 18 to 21, 2025, Yuantian Bio will showcase at the 31st China Plastics Recycling and Reuse Conference (Spring) China Replas 2025 in Zhangjiagang, Jiangsu, engaging in extensive and in-depth discussions with industry professionals about enzyme-based PET recycling technology products.

Core proprietary technologies and processes are the foundation of SourceSky Biotechnology. In recent years, SourceSky Biotechnology has expanded its research and development in enzyme modification and process development. Researchers have redesigned and modified the IsPETase enzyme to enhance both its activity and thermal stability; they have redesigned and modified the PET hydrolase LCC-ICCG to boost its catalytic activity; they have conducted a semi-rational design on the PET hydrolase PHL7 to improve its activity and thermal stability; and they have developed new processes to enhance the catalytic effect of PET hydrolases. The specific research results are as follows:
— 1 — Design and Modification of IsPETase Enzyme
The research team at Source天地 Biotech conducted rational design on PET hydrolase IsPETase to enhance its thermal stability. Through protein backbone structure analysis, they selected two flexible regions with considerable fluctuations for modification, aiming to regulate stability through electrostatic interactions.
Specifically, in Bioresour. Technol., 2022, 364: 128026, Asp157 located on the β6-β7 loop was selected as the target residue, and site-directed mutations were performed at Ser92 on the α3 helix and Ile139 on the α4 helix to construct salt bridges between these sites. This was aimed at enhancing the interactions between secondary structures to stabilize the fluctuating flexible region, thereby improving the overall stability of the enzyme and increasing its selectivity for the product TPA (Figure 1).
Technical personnel performed a semi-saturation mutation at the Ser92 site on the α3 helix and further screened the IsPETaseS92P/D157A mutant through a semi-rational design method. Under the condition of mutating only two sites, the stability achieved was the same as that reported in the literature with more than 10 mutations.
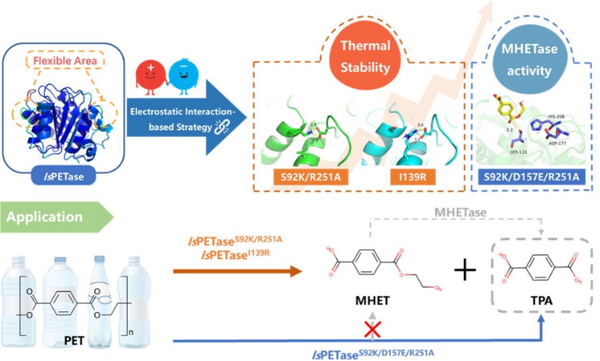
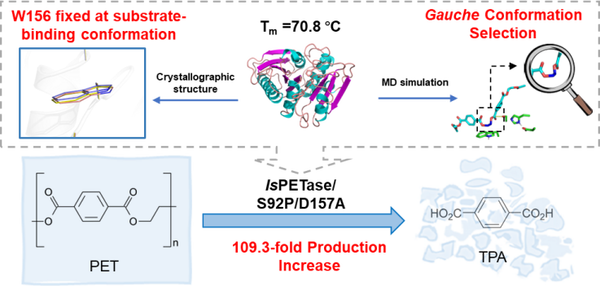
Figure 1 Rational design to enhance the thermal stability/selectivity/activity of PET hydrolase based on (semi) rational design.
-2- Design and Modification of PET Hydrolase LCC-ICCG
The researchers conducted rational design on the PET hydrolase LCC-ICCG to enhance its catalytic activity. By analyzing the active site of the protein, they selected nearby sites for modification, aiming to achieve catalytic activity regulation based on optimized interactions.
Specifically, in Biosur Technol., 2024, 406: 130929, hydrophobic modification and charge optimization were performed on amino acids near the active pocket to obtain the optimal single-point mutation (H183Y). Additionally, evolutionary analysis was used to enhance long-range interactions to improve the flexibility of the binding pocket (L124G/S29A), thereby alleviating the epistatic effects caused by the combination mutations and enhancing catalytic activity (Fig. 2).
In J. Hazard. Mater., 2025, 490: 137837, researchers emphasized the important role of the β8-α6 loop in the class I PET-degrading enzyme ICCG based on structural analysis. By combining rational design with simulation, they screened the LCC-ICCGH183Y/L202I/I208T/T153A mutant, which not only improved structural stability but also enhanced PET degradation activity by 3.46 times, outperforming other reported LCC-ICCG mutants.
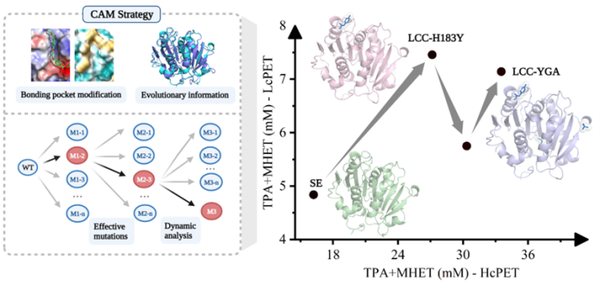
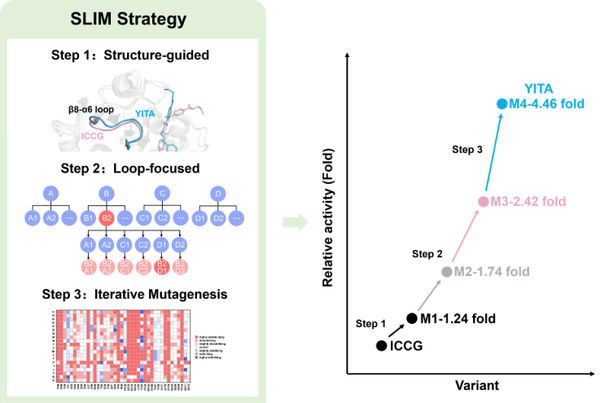
Figure 2 Rational design to enhance the catalytic activity of PET hydrolase based on structural analysis.
— 3 — Design and Modification of PHL7 PET Hydrolase
Researchers conducted semi-rational design on PET hydrolase PHL7 to enhance its activity and thermal stability. First, they established an energy-guided cumulative mutation strategy. Based on different molecular docking software, they analyzed the energy contributions of each residue in the complex structure after molecular dynamics simulations to identify key residue sites that play crucial roles in the binding of PHL7 to its substrate. They then performed two-step virtual screening based on folding energy and enzyme-substrate binding energy. The mutant variants obtained from the screening were validated through PET enzyme hydrolysis experiments. The results showed that compared to wild-type PHL7, the activity of 21 single-point mutants was improved. Further, by employing a cumulative mutation strategy, advantageous mutants were combined and screened. Ultimately, the highest-performing combination mutant, FlashPETase (PHL7E148K/T158P/S184E/H185Y), was obtained, which exhibited an activity 2.4 times higher than PHL7. Relevant research content has been published in Biochem. Eng. J., 2025, doi: 10.1016/j.bej.2025.109708.
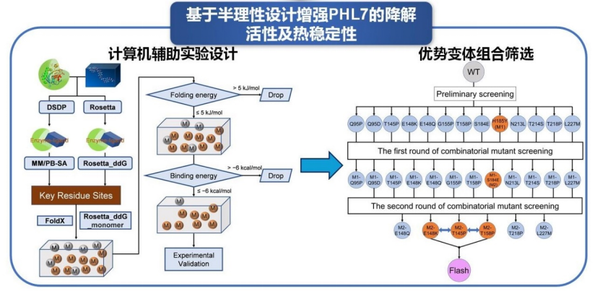
Figure 3 Enhancing the thermal stability/activity of PET hydrolase PHL7 based on semi-rational design.
- 4 - Catalytic Applications of PET Hydrolase
In the PET enzymatic degradation process,针对高结晶度底物的限制,researchers in Bioresour. Technol., 2025, 416: 131759 developed an enzymatic degradation process based on ball milling pretreatment to achieve efficient degradation of high-crystallinity polyester fibers (Fig. 4). This process has three advantages: simple operation, mild reaction conditions, and no use of toxic or harmful chemicals. Based on this technology, the yield of terephthalic acid (TPA) in PET enzymatic degradation products was significantly increased by 23.8 times, reaching 60.9%, and the purity of TPA in the soluble compounds released was also significantly improved.
Regarding the complexity of enzyme hydrolysis products, researchers in Bioresour. Technol., 2024, 413: 131461 screened the bifunctional PET hydrolase IsPETasePA to balance its degradation efficiency for both PET and MHET, and developed an efficient PET enzymatic process to enhance the purity of TPA (Figure 5). The study found that the pH decrease caused by TPA generation hindered the complete conversion of MHET to TPA. Further catalytic mechanism analysis indicated that the change in pH induced the protonation of His208 in the catalytic triad of the PET hydrolase, disrupting the interaction between IsPETasePA and MHET, thus limiting the conversion of MHET to TPA. To overcome this limitation, the researchers combined the bifunctional IsPETasePA with pH regulation strategies to achieve high-purity TPA recovery using only a single enzyme (>99% purity).
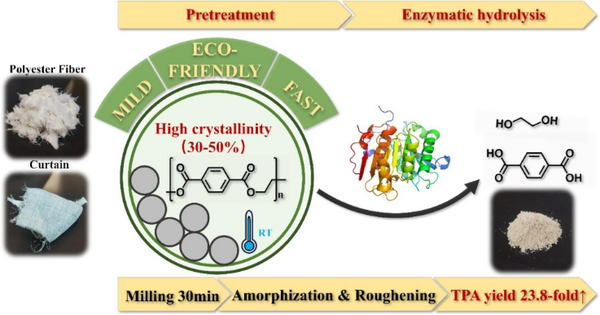
Figure 4 Development of an efficient/simple/eco-friendly enzymatic hydrolysis process for PET based on substrate pretreatment
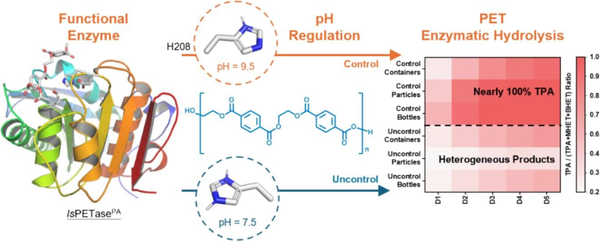
Figure 5 High-purity PET enzymatic hydrolysis product recovery process based on bifunctional hydrolase and pH regulation
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






