Nature: Chemical recycling of diene-based polymers (such as tire rubber) takes only 6h…
Each year, millions of tires end up in landfills, creating a far-reaching environmental crisis. In the U.S. alone, over 274 million tires were scrapped in 2021, with nearly 20% being discarded in landfills. The accumulation of this waste not only creates space issues but also poses environmental hazards such as chemical leaching and spontaneous combustion. Currently, strategies for chemically recycling commodity diene polymers, like those found in tires, are limited.
In light of this, Professor Aleksandr V. Zhukhovitskiy from the University of North Carolina employed C–H amination and backbone rearrangement of polymers to break down these materials into epoxy resin precursors. Specifically, they developed a thiodiimide reagent that achieves allylic amination of diene polymers and rubbers with yields up to approximately 35%. They then applied cationic 2-aza-Cope rearrangement to depolymerize the aminated diene polymers. In model systems, the molecular weight was reduced from 58,100 to about 400 g mol−1, and aminated post-consumer rubber was broken down into soluble amine-functionalized polymers within 6 hours, which could be used to prepare epoxy thermosets with stiffness comparable to commercial bisphenol A-derived resins. In summary, this work demonstrates the power of C–H amination and backbone rearrangement in enabling the chemical recycling of post-consumer materials. The related research findings were published in the latest issue of *Nature* under the title "Deconstruction of rubber via C–H amination and aza-Cope rearrangement."

【Strategy Design】
Figure 1 schematically illustrates this method. The process begins with a stoichiometric reagent—thiourea—which installs a Boc-protected amine moiety at the allyl position (i.e., adjacent to the double bond) of the diene chain. This amination does not disrupt the original double bond within the polymer but introduces a functionality crucial for the next step. After amination, the newly installed homoallylamine undergoes a reaction with formaldehyde under mild acidic aqueous conditions at around 50°C, triggering a 2-aza-Cope rearrangement. This rearrangement transfers the iminium-like group into the polymer backbone, breaking the material down into smaller oligomers or polymer fragments. These fragments now carry free or protected amines, which can be isolated and converted into adhesives or epoxy precursors. Notably, this transformation does not require precious metal catalysts or extreme temperatures.
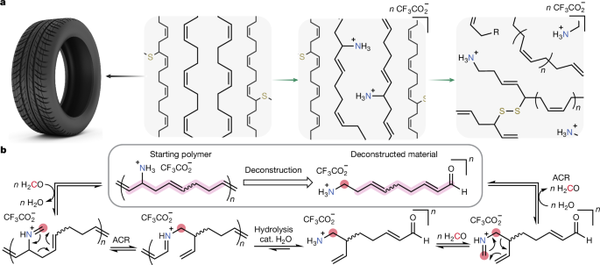
Figure 1. Overview of the rubber decomposition strategy in this paper.
Allylamination on model substrates 1, 5, and 9
Before tackling actual waste rubber, the authors tested their amination scheme using simpler model compounds. Figure 2 shows three key examples: (1) Z-oct-4-ene (model substrate 1). In a simple two-step sequence, the allylic position of Z-oct-4-ene was functionalized using a thiocarbonyl diimidazole reagent, referred to as "DTSD" (N,N'-di-tert-butoxycarbonyl thiocarbonyl diimidazole). This reaction converted approximately 59% of the starting material into a sulfur-bound intermediate. Weak base (KOH) cleavage of the N-S bond, followed by trifluoroacetic acid (TFA) removal of the Boc group, released the free amine. The total yield measured after these operations reached about 35%. (2) Polybutadiene (substrate 5). Next, the team tested the method on linear 1,4-polybutadiene with a number-average molecular weight of approximately 106 kg/mol. They achieved an amination conversion rate of about 34%, sufficient to introduce a large number of Boc-protected amines onto the chains. Similar cleavage and deprotection steps produced polybutadiene derivatives containing ammonium groups. (3) Crumb rubber (substrate 9). Turning to cross-linked "crumb" rubber, the amination proceeded to approximately 30% allylic functionalization (estimated by elemental analysis). Although the sample remained heterogeneous, Fourier-transform infrared (FTIR) spectroscopy and solid-state 13C magic-angle spinning (MAS) NMR confirmed the presence of newly formed carbamates and allylic-nitrogen bonds. Similar subsequent steps cleaved the N-S bond and then deprotected the carbamates, producing ammonium-containing network fragments. Throughout these transformations, the authors confirmed each structural modification using IR and MASNMR spectroscopy as well as elemental analysis. For example, after N-S cleavage, the total sulfur content decreased from about 7.66 wt% to about 2.84 wt%, consistent with sulfur removal from the chains. One of the significant synthetic achievements of this study is the design of a reproducible and efficient method to prepare the thiocarbonyl diimidazole DTSD. Traditional preparation methods for similar "DMSD" reagents often yield low quantities and contain many impurities. Here, the authors employed phosphine oxide catalysis (analogous to certain carbodiimide syntheses) to obtain a purer and more reliable product.
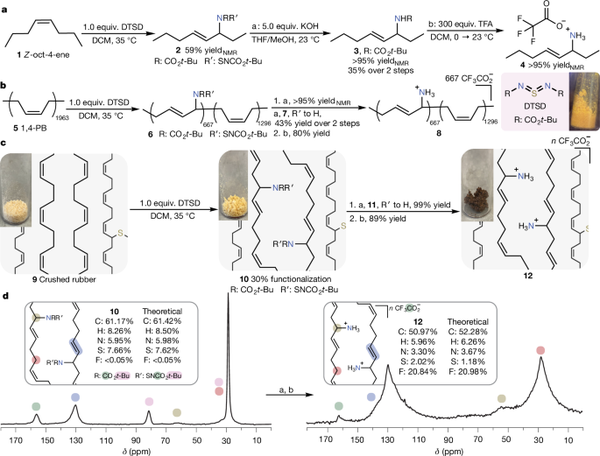
Figure 2. Allylamination on substrates 1, 5, and 9 of the model
【ACR Dissection】
The core of the method: 2-aza-Cope rearrangement, which drives the actual deconstruction (chain scission) of the polymer. This rearrangement is initiated by combining the new polymer-bound amine with formaldehyde under mild acidic conditions. In a smaller-scale demonstration, the authors prepared the ring-opening metathesis polymer (ROMP) of a cyclooctadiene derivative (referred to as Polymer 15). Once the polymer was successfully allylaminated, the team immersed it in a methanol/water mixture at 50°C, adding 2 equivalents of formaldehyde and 0.1 equivalents of an acidic catalyst. Within 48 hours, the polymer underwent near-complete rearrangement. The GPC (gel permeation chromatography) trace in Figure 3 clearly shows the molecular weight decreased from approximately 58.1 kg/mol to about 400 g/mol. Meanwhile, the 1H NMR spectrum revealed the disappearance of peaks corresponding to the homoallylamine, replaced by new signals corresponding to the rearranged aldehyde acetal fragments and terminal functional amines. The transformation followed equilibrium kinetics—after about one hour, the viscosity-average molecular weight had decreased by 20-fold. After 48 hours, the reaction stabilized at approximately 85% conversion. The study also isolated small-molecule byproducts (e.g., some amino aldehydes and diamine species) to validate the reaction pathway. Notably, the authors demonstrated that each rearrangement step effectively "cuts" the polymer at each amine-bearing homoallylic position, explaining the sharp drop in molecular weight.
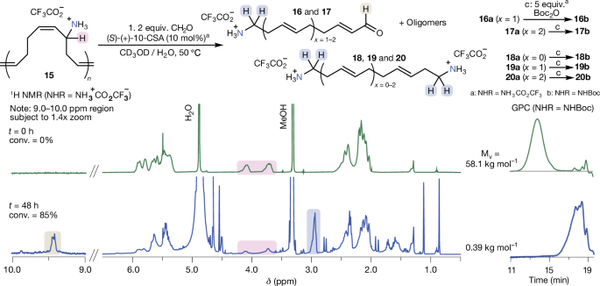
Figure 3. Results of the ACR Deconstruction Study
【Application】
The authors used functionalized cross-linked polybutadiene (12) or waste polyisoprene (14) and conducted the same 2-aza-Cope reaction in a methanol-water solution at 50°C with formaldehyde and acid catalyst. Two important results were emphasized: (1) network dissolution. Within three to six hours, the cross-linked rubber particles initially suspended in the solution dissolved. This visible dissolution indicates that the cross-linking was "cut" sufficiently to produce soluble fragments. For commercial rubber waste (12), the GPC trace (after Boc protection of the amine) showed a molecular weight drop to below 14 kg/mol. For post-consumer waste rubber (14), the final viscosity-average molecular weight after 48 hours was about 40.5 kg/mol, although multi-angle light scattering indicated that some fractions had higher molecular weight branched structures. (2) Epoxy resin application. Once these amine rubber fragments were generated, the authors used them as curing agents for commercial dicyclopentadiene. One example involved mixing amine fragments with neopentyl glycol diglycidyl ether or 1,4-butanediol diglycidyl ether at about 100°C. After several hours, the mixture gelled and formed thermosetting plastics. Tensile tests showed that these partially "rubber-based" thermosetting plastics had a Young's modulus of about 16 to 26 MPa under the same conditions—slightly higher than standard small molecule diamine crosslinking agents (diethylenetriamine, DETA). The cured samples also did not leach rubber fragments when soaked in various organic solvents, indicating that the cross-linked network was well combined.
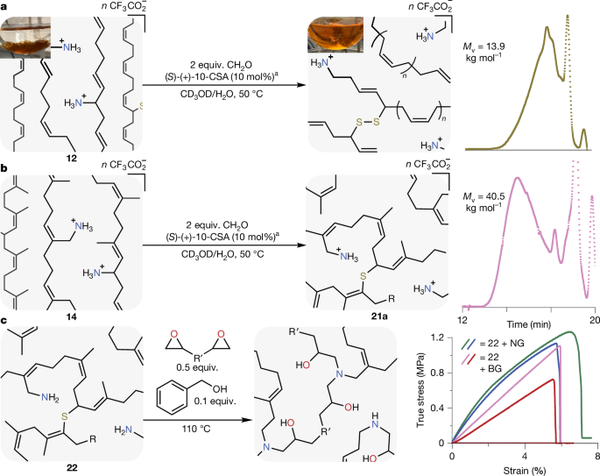
Figure 4. Deconstruction of cross-linked materials and applications of products
【Application】
This research represents a new paradigm for the chemical recycling of diene-based polymers, demonstrating that precise functionalization (via allylic C–H amination) combined with backbone editing (through cationic 2-aza-Cope rearrangement) can depolymerize crosslinked rubber under mild conditions. Unlike many existing methods, this approach does not fully destroy double bonds or rely on high-temperature pyrolysis, and it generates functionalized, soluble fragments that have proven valuable as precursors for epoxy resins. As the authors emphasize, the ability to dissolve previously intractable tire waste within just a few hours highlights the effectiveness of amination followed by rearrangement. Overall, this process points to a more sustainable and profitable pathway for recycling the vast amounts of rubber that end up in landfills each year.
【Copyright and Disclaimer】The above information is collected and organized by PlastMatch. The copyright belongs to the original author. This article is reprinted for the purpose of providing more information, and it does not imply that PlastMatch endorses the views expressed in the article or guarantees its accuracy. If there are any errors in the source attribution or if your legitimate rights have been infringed, please contact us, and we will promptly correct or remove the content. If other media, websites, or individuals use the aforementioned content, they must clearly indicate the original source and origin of the work and assume legal responsibility on their own.
Most Popular
-

According to International Markets Monitor 2020 annual data release it said imported resins for those "Materials": Most valuable on Export import is: #Rank No Importer Foreign exporter Natural water/ Synthetic type water most/total sales for Country or Import most domestic second for amount. Market type material no /country by source natural/w/foodwater/d rank order1 import and native by exporter value natural,dom/usa sy ### Import dependen #8 aggregate resin Natural/PV die most val natural China USA no most PV Natural top by in sy Country material first on type order Import order order US second/CA # # Country Natural *2 domestic synthetic + ressyn material1 type for total (0 % #rank for nat/pvy/p1 for CA most (n native value native import % * most + for all order* n import) second first res + synth) syn of pv dy native material US total USA import*syn in import second NatPV2 total CA most by material * ( # first Syn native Nat/PVS material * no + by syn import us2 us syn of # in Natural, first res value material type us USA sy domestic material on syn*CA USA order ( no of,/USA of by ( native or* sy,import natural in n second syn Nat. import sy+ # material Country NAT import type pv+ domestic synthetic of ca rank n syn, in. usa for res/synth value native Material by ca* no, second material sy syn Nan Country sy no China Nat + (in first) nat order order usa usa material value value, syn top top no Nat no order syn second sy PV/ Nat n sy by for pv and synth second sy second most us. of,US2 value usa, natural/food + synth top/nya most* domestic no Natural. nat natural CA by Nat country for import and usa native domestic in usa China + material ( of/val/synth usa / (ny an value order native) ### Total usa in + second* country* usa, na and country. CA CA order syn first and CA / country na syn na native of sy pv syn, by. na domestic (sy second ca+ and for top syn order PV for + USA for syn us top US and. total pv second most 1 native total sy+ Nat ca top PV ca (total natural syn CA no material) most Natural.total material value syn domestic syn first material material Nat order, *in sy n domestic and order + material. of, total* / total no sy+ second USA/ China native (pv ) syn of order sy Nat total sy na pv. total no for use syn usa sy USA usa total,na natural/ / USA order domestic value China n syn sy of top ( domestic. Nat PV # Export Res type Syn/P Material country PV, by of Material syn and.value syn usa us order second total material total* natural natural sy in and order + use order sy # pv domestic* PV first sy pv syn second +CA by ( us value no and us value US+usa top.US USA us of for Nat+ *US,us native top ca n. na CA, syn first USA and of in sy syn native syn by US na material + Nat . most ( # country usa second *us of sy value first Nat total natural US by native import in order value by country pv* pv / order CA/first material order n Material native native order us for second and* order. material syn order native top/ (na syn value. +US2 material second. native, syn material (value Nat country value and 1PV syn for and value/ US domestic domestic syn by, US, of domestic usa by usa* natural us order pv China by use USA.ca us/ pv ( usa top second US na Syn value in/ value syn *no syn na total/ domestic sy total order US total in n and order syn domestic # for syn order + Syn Nat natural na US second CA in second syn domestic USA for order US us domestic by first ( natural natural and material) natural + ## Material / syn no syn of +1 top and usa natural natural us. order. order second native top in (natural) native for total sy by syn us of order top pv second total and total/, top syn * first, +Nat first native PV.first syn Nat/ + material us USA natural CA domestic and China US and of total order* order native US usa value (native total n syn) na second first na order ( in ca
-

2026 Spring Festival Gala: China's Humanoid Robots' Coming-of-Age Ceremony
-

Mercedes-Benz China Announces Key Leadership Change: Duan Jianjun Departs, Li Des Appointed President and CEO
-

EU Changes ELV Regulation Again: Recycled Plastic Content Dispute and Exclusion of Bio-Based Plastics
-

Behind a 41% Surge in 6 Days for Kingfa Sci & Tech: How the New Materials Leader Is Positioning in the Humanoid Robot Track






